LBF17307HO02
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6201 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF17307HO02 |
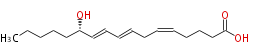
| 12S-Hydroxy- (5Z,8E,10E) -heptadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 12S-Hydroxy- (cis-5,trans-8,trans-10) -heptadecatrienoic acid | |
| |
| HHT | |
| Formula | C17H28O3 |
| Exact Mass | 280.203844762 |
| Average Mass | 280.40242 |
| SMILES | CCCCC[C@H](O)C=CC=CCC=CCCCC(O)=O |
| Physicochemical Information | |
| METHYL ESTER ; [ α ]25 D =+7.5°(C=0.2, CHLOROFORM) Nicolaou_KC et al. | |
| DIETHYL ETHER HambergMet al. | |
Nicolaou_KC et al.  | |
| When prostaglandin H2 reacts with thromboxane A synthase and the endoperoxide moiety is cleaved, the production of thromboxane A2 is accompanied by the formation of 12(S)-hydroxy-5,8,10-heptadecatrienoic acid in an almost equimolar amount liberating malondialdehyde Hamberg_M et al.. This compound is also a product of non-enxymatic degradation of prostaglandin H2 Nugteren_DH et al.. | |
| The compound stimulates chemotactic and chemokinetic activities of human polymorphonuclear leukocytes Goetzl_EJ et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 366(M+), 335, 295, 276, 225, 173, (128) HambergMet al. METHYL ESTER ; 298(M+), 224 Nicolaou_KC et al. |
| UV Spectra | METHYL ESTER ; ETHANOL : 232nm( ε 33,400) HambergMet al.. METHANOL : 240nm Nicolaou_KC et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : δ 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH3), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH2 and CH3) Nicolaou_KC et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|