LBF18203HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01040040 | |LipidMaps=LMFA01040040 | ||
|SysName=Methyl-10,12-Epidioxy-9-Hydroperoxy-13,15-Octadecadienoate | |SysName=Methyl-10,12-Epidioxy-9-Hydroperoxy-13,15-Octadecadienoate | ||
|Common Name=&&Methyl-10,12-Epidioxy-9-Hydroperoxy-13,15-Octadecadienoate&& | |||
|Mass Spectra=GC-EI-MS(after reduction(PH3P) and TMS)[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]]: m/e=397[M-CH3]; 259[SMTO=CH(CH2)7COOCH3]; GC-EI-MS(after reduction with NaBH4 or KI andTMS)[[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=259[SMTO=CH(CH2)7COOCH3]; 183[SMTO=CH-CH=CH-CH=CH-CH2CH3] | |Mass Spectra=GC-EI-MS(after reduction(PH3P) and TMS)[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]]: m/e=397[M-CH3]; 259[SMTO=CH(CH2)7COOCH3]; GC-EI-MS(after reduction with NaBH4 or KI andTMS)[[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BPS:Garwood_RF:Weedon_BCL:,Lipids,1977,12,1055}}]]: m/e=259[SMTO=CH(CH2)7COOCH3]; 183[SMTO=CH-CH=CH-CH=CH-CH2CH3] | ||
|UV Spectra=Conjugated diene: <FONT FACE="Symbol">l</FONT>max=231-236nm [[Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84|{{RelationTable/GetFirstAuthor|Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84}}]][[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]][[Reference:Coxon_DT:Price_KR:Chan_HWS:,Chem. Phys. Lipids,1981,28,365|{{RelationTable/GetFirstAuthor|Reference:Coxon_DT:Price_KR:Chan_HWS:,Chem. Phys. Lipids,1981,28,365}}]][[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]] | |UV Spectra=Conjugated diene: <FONT FACE="Symbol">l</FONT>max=231-236nm [[Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84|{{RelationTable/GetFirstAuthor|Reference:Toyoda_I:Terao_J:Matsushita_S:,Lipids,1982,17,84}}]][[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1981,16,439}}]][[Reference:Coxon_DT:Price_KR:Chan_HWS:,Chem. Phys. Lipids,1981,28,365|{{RelationTable/GetFirstAuthor|Reference:Coxon_DT:Price_KR:Chan_HWS:,Chem. Phys. Lipids,1981,28,365}}]][[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]] | ||
Revision as of 00:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8056 |
| LipidMaps | LMFA01040040 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18203HP01 |
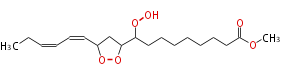
| Methyl-10,12-Epidioxy-9-Hydroperoxy-13,15-Octadecadienoate | |
|---|---|
| |
| Structural Information | |
| Methyl-10,12-Epidioxy-9-Hydroperoxy-13,15-Octadecadienoate | |
| |
| Formula | C19H32O6 |
| Exact Mass | 356.219888756 |
| Average Mass | 356.45378 |
| SMILES | C(O1)(CC(C=CC=CCC)O1)C(OO)CCCCCCCC(=O)OC |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction(PH3P) and TMS) Neff_WE et al.: m/e=397[M-CH3]; 259[SMTO=CH(CH2)7COOCH3]; GC-EI-MS(after reduction with NaBH4 or KI andTMS) Frankel_EN et al.: m/e=259[SMTO=CH(CH2)7COOCH3]; 183[SMTO=CH-CH=CH-CH=CH-CH2CH3] |
| UV Spectra | Conjugated diene: lmax=231-236nm ToyodaIet al. Neff_WE et al. Coxon_DT et al. Neff_WE et al. |
| IR Spectra | OOH group: 3520 cm-1[free], 3700-3100cm-1[bonded]; olefinic protons: 3020-3000cm-1; CONJUGATE TRANS, CIS DIENES: 990-980cm-1, 955-947cm-1 Neff_WE et al. Coxon_DT et al. Neff_WE et al. Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al. Neff_WE et al. Coxon_DT et al.: C9: 3.92-4.21; C10: 4.45-4.47; C11: 2.19-2.47, 2.81-2.87; C12: 4.77-4.80; C13: 5.57-5.62; C14: 6.65-6.68; C15: 5.96-6.65; C16: 5.50-5.55; OOH: 9.05-9.56ppm; J13-14=15Hz[C13-14 : trans]; J15-16=10Hz[C15-16: cis]13C-NMR Neff_WE et al. Neff_WE et al.: C9: 86.0; C10, 12: 83.8, 83.0 |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|