LBF18207HP01
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8002 |
| LipidMaps | LMFA01040001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HP01 |
| 13-HPODE | |
|---|---|
| |
| Structural Information | |
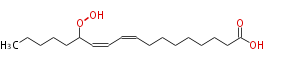
| 13-Hydroperoxy-9,11-octadecadienoic acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC(OO)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Auto oxidation of methyl linoleate Frankel_EN Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al.. Oxidation of methyl linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN . Oxidation of linoleic acid by lipoxygenase Mathuo_M Wakabayashi_T . Production mechanism (auto oxidation): bis-allylic hydrogen at C11. | |
| Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously. Logani_MK et al. Sevanian_A et al. Fujimoto_K | |
| Spectral Information | |
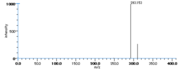
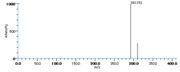
| Mass Spectra | GC-EI-MS(after methanolysis, reduction and trimethylsilylation) Frankel_EN et al. KleimanRet al. Frankel_EN et al. Gardner_HW et al. HambergM: m/e= 382[M], 292[M-HOTMS], 311[M-(CH2)4CH3] standard peak,225[M-(CH2)7COOCH3] / GC-EI-MS(after methylation, reduction and hydrogenation) Chan_HWS DolevAet al. Zimmerman_DC et al.: m/e= 243[CH(OH)(CH2)11COOCH3], 214[(CH2)11COOCH3+H], 211[C(OH(CH2)11CO] |
| UV Spectra | Trans, cis isomer: λ max= 236nm, ε = 26000, trans, trans isomer: λ max= 233nm, ε =28600 (025/027/028/029/036) |
| IR Spectra | Methyl ester Chan_HW et al. Cannon_JA et al. Privett_OS et al. Sephton_HH et al. Graveland_A_ Privett_OS et al. Gardner_HW et al.: cis, trans isomer: 986 and 949cm-1, trans, trans isomer: 989cm-1, OOH group: 3550cm-1 |
| NMR Spectra | 1H-NMR Chan_HW et al. Frankel_EN et al.: C9-13 (5.45-6.53ppm), C13 (4.37ppm), J9-10= 11Hz (cis),J11-12=15Hz (trans) /1H-NMR (after methanolyzation and reduction ) Gardner_HW et al. Neff_WE et al.: cis, trans isomer : olefinic protons(5.91ppm), C8 (2.10ppm), C13(4.15ppm), trans, trans isomer: olefinic protons (5.41ppm), C8 (2.07ppm) |
| Other Spectra | |
| Chromatograms | |