LBF20107PG02
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1430 |
| LipidMaps | LMFA03010012 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG02 |
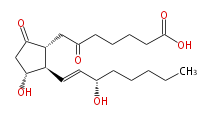
| 6-KETO-PROSTAGLANDIN E1 | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -5-oxocyclopentan-1 (R) -yl ] -6-oxoheptanoic acid | |
| |
| 6-KETO-PGE1 | |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@@H](CC(=O)CCCCC(O)=O)1)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 65°C Tanaka_T et al. | |
| [a]XD20=-50°(C=1.55, METHANOL) TanakaTet al. | |
| METHANOL, CHLOROFORM TanakaTet al. | |
| There were reports of prolonged biological activity of chemically unstable prostaglandin I2, which suggested a possible transformation of prostaglandin I2 to a more stable metabolite with potent bioactivity Wong_PY et al.. Further investigations led to the discovery of 6-keto-prostaglandin E1. | |
Tanaka_T et al.  | |
| A potential role of 9-hydroxyprostaglandin dehydrogenase was demonstrated in the transformation of prostaglandin I2 to 6-keto-prostaglandin E1 Wong_PY et al.. | |
| 6-Keto-prostaglandin E1 is a stable metabolite. The compound inhibits platelet aggregation with activity comparable or greater than prostaglandin D2 although less potent than prostaglandin I2. Its vasodilatory and hypotensive activities, bronchodilatory property, and inhibition of gastric acid secretion were reported Moore_PK et al.. | |
| Spectral Information | |
| Mass Spectra | DIRECT EXPOSURE AMMONIA CI, POSITIVE : 386(M++18), 368(M+), 351, 350, 244, 136. NEGATIVE : 368(M+) ,350, 338, 332 Cepa_SR et al. |
| UV Spectra | |
| IR Spectra | KBr: n 3400, 1740, 1720, 1710, 1245, 1160, 1075, 970 cm-1 TanakaTet al. |
| NMR Spectra | 1H-NMR(CDCl3) : d 5.58(m, 2H, 13,14-CH), 4.09(m, 1H, 11-CH), 4.02(m, 1H, 15-CH), 2.7(2H, 7-CH), 2.69(IH, 10b-CH), 2.45-2.47(m, 3H, 5,8,12-CH), 2.29(1H, 10a-CH), 2.28(2H, 2-CH), 1.58 - 1.32(12H), 0.91(t, 3H, 20-CH3) KotovychGet al.. METHYL ESTER ; 13C-NMR(CDCl3) : 216.6, 208.4, 173.8, 138.0, 126.5, 72.3, 51.5, 44.6, 45.7, 42.4, 37.6, 33.8, 31.8, 25.2, 24.5, 23.4, 22.6, 14.0 TanakaTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|