LBF20406AM37: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
|Optical=[ alpha ]^{25}_4 = +10.9° [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |Optical=[ alpha ]^{25}_4 = +10.9° [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|NMR Spectra=^1 H NMR (200 MHz, CDCl3) delta (TMS)5.57 (m, 1H), 5.47-5.30 (m, 8H), 4.14-4.02 (m, 1H), 3.71-3.48 (m, 2H), 2.84-2.81 (m, 6H), 2.24-2.01 (m, 6H), 1.77-1.65 (m, 2H), 1.39-1.26 (m, 6H), 1.17 (d, J=3.46Hz, 3H), 0.89 (t, J=6.12Hz, 3H) [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |NMR Spectra=^1 H NMR (200 MHz, CDCl3) delta (TMS)5.57 (m, 1H), 5.47-5.30 (m, 8H), 4.14-4.02 (m, 1H), 3.71-3.48 (m, 2H), 2.84-2.81 (m, 6H), 2.24-2.01 (m, 6H), 1.77-1.65 (m, 2H), 1.39-1.26 (m, 6H), 1.17 (d, J=3.46Hz, 3H), 0.89 (t, J=6.12Hz, 3H) [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|Chromatograms=Rf 0.3(5% MeOH/CHCl3) [[Reference: | |Chromatograms=Rf 0.3(5% MeOH/CHCl3) [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=This compound was synthesized from arachidonic acid and (R)-(-)-2-amino-l-propanol in 69% yield. [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |Chemical Synthesis=This compound was synthesized from arachidonic acid and (R)-(-)-2-amino-l-propanol in 69% yield. [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
Latest revision as of 06:48, 8 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7054 |
| LipidMaps | LMFA08020040 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406AM37 |
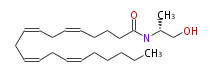
| N- (R) -1-Methyl-2-hydroxyethylarachidonoylamide | |
|---|---|
| |
| Structural Information | |
| N-1R-Methyl-2-hydroxyethyl- (cis-5,cis-8,cis-11,cis-14) -eicosatetraenoylamine | |
| |
| Formula | C23H39NO2 |
| Exact Mass | 361.298079497 |
| Average Mass | 361.5613 |
| SMILES | C(CCC(=O)N[C@@H](CO)C)C=CCC=CCC=CCC=CCCCCC |
| Physicochemical Information | |
| colorless liquid Abadji_V et al.. | |
| [ α ]25 4 = +10.9° AbadjiVet al. | |
| This compound was synthesized from arachidonic acid and (R)-(-)-2-amino-l-propanol in 69% yield. Abadji_V et al. | |
 Seltzman_HH et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (200 MHz, CDCl3) δ (TMS)5.57 (m, 1H), 5.47-5.30 (m, 8H), 4.14-4.02 (m, 1H), 3.71-3.48 (m, 2H), 2.84-2.81 (m, 6H), 2.24-2.01 (m, 6H), 1.77-1.65 (m, 2H), 1.39-1.26 (m, 6H), 1.17 (d, J=3.46Hz, 3H), 0.89 (t, J=6.12Hz, 3H) AbadjiVet al. |
| Other Spectra | |
| Chromatograms | Rf 0.3(5% MeOH/CHCl3) AbadjiVet al. |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|