LBF09107BC01: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA7096 | |LipidBank=DFA7096 | ||
|LipidMaps=LMFA01020128 | |LipidMaps=LMFA01020128 | ||
|SysName=3-Methyl-2- | |SysName=3-Methyl-2-nonenoic acid | ||
|Common Name=&&3-Methyl-2- | |Common Name=&&3-Methyl-2-nonenoic acid&& | ||
|Boiling Point=113 - 114°C/1mmHg [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | |Boiling Point=113 - 114°C/1mmHg [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | ||
|Refractive= | |Refractive= eta 25/D = 1.4648 [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | ||
|UV Spectra= | |UV Spectra= lambda max 219m mu [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | ||
|IR Spectra=5.90(C=O), 6.10(C=C) [[Reference:Freeman_NK:,J. Am. Chem. Soc.,1953,75,1859|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1953,75,1859}}]] | |IR Spectra=5.90(C=O), 6.10(C=C) [[Reference:Freeman_NK:,J. Am. Chem. Soc.,1953,75,1859|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1953,75,1859}}]] | ||
|Source= | |Source= | ||
Latest revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA7096 |
| LipidMaps | LMFA01020128 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF09107BC01 |
| 3-Methyl-2-nonenoic acid | |
|---|---|
| |
| Structural Information | |
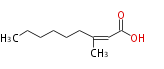
| 3-Methyl-2-nonenoic acid | |
| |
| Formula | C10H18O2 |
| Exact Mass | 170.13067981999998 |
| Average Mass | 170.24872 |
| SMILES | CCCCCCC(C)=CC(O)=O |
| Physicochemical Information | |
| 113 - 114°C/1mmHg Cason_J et al. | |
| η 25/D = 1.4648 CasonJet al. | |
| 3-Methyl-2-nonenoic acid is prepared from 3-hydroxy-3-methyl-pelargonic acid and acetanhydride Fieser_LF_et_al . | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max 219m μ CasonJet al. |
| IR Spectra | 5.90(C=O), 6.10(C=C) Freeman_NK |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|