LBF11101SC01: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA01030036 | |LipidMaps=LMFA01030036 | ||
|SysName=10-Undecenoic acid | |SysName=10-Undecenoic acid | ||
|Common Name=&&10-Hendecenoic acid&&10- | |Common Name=&&10-Hendecenoic acid&&10-Undecylenic acid&& | ||
|Melting Point=24.5°C | |Melting Point=24.5°C | ||
|Boiling Point=275°C at 25 mmHg | |Boiling Point=275°C at 25 mmHg | ||
|Density= | |Density=d^{25}_4 0.9075 | ||
| | |Refractive=1.4464 at 20°C | ||
|Solubility=soluble in ethanol and ether. slightly soluble in chloroform. insoluble in water. | |Solubility=soluble in ethanol and ether. slightly soluble in chloroform. insoluble in water.<!--0192--><!--0381--> | ||
|Source= | |||
|Chemical Synthesis=Occurs in sweat. Obtained by pyrolysis of ricinoleic acid. Preparation by bacuum distillation of castor oil:<!--3001-->/[[Reference:Perkins_GA:Cruz_AO:,J. Am. Chem. Soc.,1927,49,1070 |{{RelationTable/GetFirstAuthor|Reference:Perkins_GA:Cruz_AO:,J. Am. Chem. Soc.,1927,49,1070 }}]] Found that distillation at 400° under a pressure of 50 mm produced a distillate composed of about 40% heptaldehyde and 20% undecylenic acid. | |||
|Metabolism= | |||
|Symbol=C11:1 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 01:43, 6 September 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0075 |
| LipidMaps | LMFA01030036 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF11101SC01 |
| 10-Hendecenoic acid | |
|---|---|
| |
| Structural Information | |
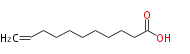
| 10-Undecenoic acid | |
| |
| C11:1 | |
| Formula | C11H20O2 |
| Exact Mass | 184.14632988399998 |
| Average Mass | 184.2753 |
| SMILES | C=CCCCCCCCCC(O)=O |
| Physicochemical Information | |
| 24.5°C | |
| 275°C at 25 mmHg | |
| d25 4 0.9075 | |
| 1.4464 at 20°C | |
| soluble in ethanol and ether. slightly soluble in chloroform. insoluble in water. | |
| Occurs in sweat. Obtained by pyrolysis of ricinoleic acid. Preparation by bacuum distillation of castor oil:/ Perkins_GA et al. Found that distillation at 400° under a pressure of 50 mm produced a distillate composed of about 40% heptaldehyde and 20% undecylenic acid. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|