LBF16000PG02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&8- [ 2 (R) - (2-Carboxyethyl) -3 (S) ,5 (R) -dihydroxycyclopentan-1 (R) -yl ] -6-oxooctanoic acid&& | |Common Name=&&8- [ 2 (R) - (2-Carboxyethyl) -3 (S) ,5 (R) -dihydroxycyclopentan-1 (R) -yl ] -6-oxooctanoic acid&& | ||
|Mass Spectra=DIMETHYL ESTER DI-TMS ETHER ; m/e 502(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 487, 412, 397, 325, 322, 291, 254, 241, 228, 217, 191, 179, 143 [[Reference:Granstrom_E:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,3398|{{RelationTable/GetFirstAuthor|Reference:Granstrom_E:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,3398}}]] | |Mass Spectra=DIMETHYL ESTER DI-TMS ETHER ; m/e 502(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 487, 412, 397, 325, 322, 291, 254, 241, 228, 217, 191, 179, 143 [[Reference:Granstrom_E:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,3398|{{RelationTable/GetFirstAuthor|Reference:Granstrom_E:Samuelsson_B:,J. Am. Chem. Soc.,1969,91,3398}}]] | ||
|Source=When prostaglandin F2<FONT FACE="Symbol">a</FONT> was administered intravenously to female subjects, 5<FONT FACE="Symbol">a</FONT>,7<FONT FACE="Symbol">a</FONT>-dihydroxy-11-keto-tetranor-prosta-1,16-dioic acid was found as a major urinary metabolite (PGF<FONT FACE="Symbol">a</FONT>-MUM) [[Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254|{{RelationTable/GetFirstAuthor|Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254}}]] | |Source=When prostaglandin F2<FONT FACE="Symbol">a</FONT> was administered intravenously to female subjects, 5<FONT FACE="Symbol">a</FONT>,7<FONT FACE="Symbol">a</FONT>-dihydroxy-11-keto-tetranor-prosta-1,16-dioic acid was found as a major urinary metabolite (PGF<FONT FACE="Symbol">a</FONT>-MUM) [[Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254|{{RelationTable/GetFirstAuthor|Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
Revision as of 11:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1521 |
| LipidMaps | LMFA03010139 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF16000PG02 |
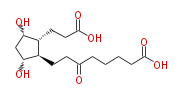
| 8- [ 2 (R) - (2-Carboxyethyl) -3 (S) ,5 (R) -dihydroxycyclopentan-1 (R) -yl ] -6-oxooctanoic acid | |
|---|---|
| |
| Structural Information | |
| 8- [ 2 (R) - (2-Carboxyethyl) -3 (S) ,5 (R) -dihydroxycyclopentan-1 (R) -yl ] -6-oxooctanoic acid | |
| |
| Formula | C16H26O7 |
| Exact Mass | 330.167853186 |
| Average Mass | 330.37343999999996 |
| SMILES | OC(=O)CCCCC(=O)CC[C@@H]([C@H](O)1)[C@@H](CCC(O)=O)[C@@H](O)C1 |
| Physicochemical Information | |
| When prostaglandin F2a was administered intravenously to female subjects, 5a,7a-dihydroxy-11-keto-tetranor-prosta-1,16-dioic acid was found as a major urinary metabolite (PGFa-MUM) Granstrom_E et al.. | |
| Spectral Information | |
| Mass Spectra | DIMETHYL ESTER DI-TMS ETHER ; m/e 502(M+), 487, 412, 397, 325, 322, 291, 254, 241, 228, 217, 191, 179, 143 GranstromEet al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|