LBF17307HO02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName= (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | |SysName= (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | ||
|Reflactive=METHYL ESTER ; [<FONT FACE=""Symbol"">a</FONT>]X<sub>D</sub><sup>25</sup> =+7.5°(C=0.2, CHLOROFORM) | |Reflactive=METHYL ESTER ; [<FONT FACE=""Symbol"">a</FONT>]X<sub>D</sub><sup>25</sup> =+7.5°(C=0.2, CHLOROFORM) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|Solubility=DIETHYL ETHER | |Solubility=DIETHYL ETHER [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | ||
|Mass Spectra=METHYL ESTER TMS ETHER ; m/e 366(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 335, 295, 276, 225, 173, (128) | |Mass Spectra=METHYL ESTER TMS ETHER ; m/e 366(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 335, 295, 276, 225, 173, (128)[[Reference:Hamberg_M:Svensson_J:Wakabayashi_T:Samuelsson_B:,Proc. Nat. Acad. Sci. USA,1974,71,345|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Svensson_J:Wakabayashi_T:Samuelsson_B:,Proc. Nat. Acad. Sci. USA,1974,71,345}}]] METHYL ESTER ; 298(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 224 [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|UV Spectra=METHYL ESTER ; ETHANOL : 232nm(<FONT FACE=""Symbol"">e</FONT> 33,400) | |UV Spectra=METHYL ESTER ; ETHANOL : 232nm(<FONT FACE=""Symbol"">e</FONT> 33,400)[[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]]. METHANOL : 240nm [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|NMR Spectra=METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE=""Symbol"">d</FONT> 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH<SUB><FONT SIZE=-1>2</FONT></SUB> and CH<SUB><FONT SIZE=-1>3</FONT></SUB>) | |NMR Spectra=METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE=""Symbol"">d</FONT> 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH<SUB><FONT SIZE=-1>2</FONT></SUB> and CH<SUB><FONT SIZE=-1>3</FONT></SUB>) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR6201 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF17307HO02 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
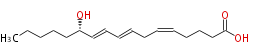
| (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | |
| Formula | C17H28O3 |
| Exact Mass | 280.203844762 |
| Average Mass | 280.40242 |
| SMILES | CCCCC[C@H](O)C=CC=CCC=CCCCC(O)=O |
| Physicochemical Information | |
| DIETHYL ETHER HambergMet al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 366(M+), 335, 295, 276, 225, 173, (128) HambergMet al. METHYL ESTER ; 298(M+), 224 Nicolaou_KC et al. |
| UV Spectra | METHYL ESTER ; ETHANOL : 232nm(e 33,400) HambergMet al.. METHANOL : 240nm Nicolaou_KC et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : d 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH3), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH2 and CH3) Nicolaou_KC et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|