LBF17307HO02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName= (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | |SysName= (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | ||
|Common Name=&& (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid&& | |||
|Reflactive=METHYL ESTER ; [<FONT FACE=""Symbol"">a</FONT>]X<sub>D</sub><sup>25</sup> =+7.5°(C=0.2, CHLOROFORM) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | |Reflactive=METHYL ESTER ; [<FONT FACE=""Symbol"">a</FONT>]X<sub>D</sub><sup>25</sup> =+7.5°(C=0.2, CHLOROFORM) [[Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131|{{RelationTable/GetFirstAuthor|Reference:Nicolaou_KC:Stylianides_NA:Ramphal_JY:,J. Chem. Soc. Perkin Trans. I,1989,,2131}}]] | ||
|Solubility=DIETHYL ETHER [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | |Solubility=DIETHYL ETHER [[Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1974,71,3400}}]] | ||
Revision as of 00:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR6201 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF17307HO02 |
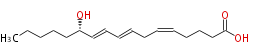
| (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| (S) , (Z,E,E) -12-Hydroxy-5,8,10-heptadecatrienoic acid | |
| |
| Formula | C17H28O3 |
| Exact Mass | 280.203844762 |
| Average Mass | 280.40242 |
| SMILES | CCCCC[C@H](O)C=CC=CCC=CCCCC(O)=O |
| Physicochemical Information | |
| DIETHYL ETHER HambergMet al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 366(M+), 335, 295, 276, 225, 173, (128) HambergMet al. METHYL ESTER ; 298(M+), 224 Nicolaou_KC et al. |
| UV Spectra | METHYL ESTER ; ETHANOL : 232nm(e 33,400) HambergMet al.. METHANOL : 240nm Nicolaou_KC et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : d 6.17(dd, J=15.11, 10.36Hz, 1H, 10-CH), 6.04(dd, J=15,05, 10.52Hz, 1H, 9-CH), 5.66(dt, J=15.16, 6.48Hz, 1H), 5.60(dd, J=17.17, 7.04Hz, 1H, 11-CH), 5.42(m, 2H, 5-CH, 6-CH), 4.1(m, 1H, 12-CH), 3.66(s, 3H, COOCH3), 2.81(m, 2H, 7-CH), 2.36(t, J=7.51Hz, 2H, 2-CH), 2.1-0.85(m, 16H, CH2 and CH3) Nicolaou_KC et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|