LBF18102HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Lipid/Header}} | |||
{{Metabolite | {{Metabolite | ||
| Line 10: | Line 12: | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]: C2: 2.3ppm; C9: 3.88ppm[C9-10 erythro], 4.17ppm[C9-10 threo]; C10, 12, 13, 15: 4.47ppm; C11, 14: 2.1-2.8ppm; C16: 5.88ppm; C17: 5.35ppm; C18: 1.73ppm; OOH: 8.73ppm[C9-10 erythro], 9.20ppm[C9-10 threo] [[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]]: C2: 2.3ppm; C9: 3.88ppm[C9-10 erythro], 4.17ppm[C9-10 threo]; C10, 12, 13, 15: 4.47ppm; C11, 14: 2.1-2.8ppm; C16: 5.88ppm; C17: 5.35ppm; C18: 1.73ppm; OOH: 8.73ppm[C9-10 erythro], 9.20ppm[C9-10 threo] [[Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Weisleder_D:,Lipids,1982,17,780}}]] | ||
}} | }} | ||
{{Lipid/Footer}} | |||
Revision as of 13:00, 26 July 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8067 |
| LipidMaps | LMFA01040051 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18102HP01 |
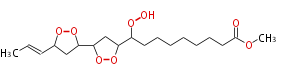
| Methyl-10,12,13,15-Bisepidioxy-9-Hydroperoxy-16-Octadecenoate | |
|---|---|
| |
| Structural Information | |
| Methyl-10,12,13,15-Bisepidioxy-9-Hydroperoxy-16-Octadecenoate | |
| |
| Formula | C19H32O8 |
| Exact Mass | 388.20971799999995 |
| Average Mass | 388.45258 |
| SMILES | C(O1)(C(OO)CCCCCCCC(=O)OC)CC(C(O2)CC(C=CC)O2)O1 |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction(PH3P) and TMS-derivatization) Neff_WE et al.: m/e=259[SMTO=CH(CH2)7COOCH3]; 185[M-259]; GC-EI-MS(after reduction, hydrogenation, and TMS-derivatization)(105): m/e=261[SMTO=CHCH2CH(OTMS)(CH2)2CH3]; 259[SMTO=CH(CH2)7COOCH3] |
| UV Spectra | |
| IR Spectra | OOH group: 3700-3150cm-1[bonded], 3530cm-1[free], isolated trans unsaturation: 960cm-1 Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al.: C2: 2.3ppm; C9: 3.88ppm[C9-10 erythro], 4.17ppm[C9-10 threo]; C10, 12, 13, 15: 4.47ppm; C11, 14: 2.1-2.8ppm; C16: 5.88ppm; C17: 5.35ppm; C18: 1.73ppm; OOH: 8.73ppm[C9-10 erythro], 9.20ppm[C9-10 threo] Neff_WE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|