LBF18106EO01: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA01070011 | |LipidMaps=LMFA01070011 | ||
|SysName=9,10-Epoxy-12-octadecenoic acid | |SysName=9,10-Epoxy-12-octadecenoic acid | ||
|Common Name=&&9,10- | |Common Name=&&9,10-EODE&& | ||
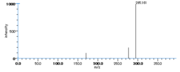
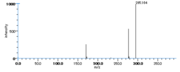
|Mass Spectra=GC-EI-MS[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]: m/e= 310[M], 279[M-OCH3], 199[M-CH3(CH2)4CH=CHCH2], 153[M-(CH2)7-COOCH3], GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]] | |Mass Spectra=GC-EI-MS[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]: m/e= 310[M], 279[M-OCH3], 199[M-CH3(CH2)4CH=CHCH2], 153[M-(CH2)7-COOCH3], GC-EI-MS(after solvolysation-trimethylsilylation in MeOH)[[Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971|{{RelationTable/GetFirstAuthor|Reference:Wu_GS:Stein_RA:Mead_JF:,Lipids.,1977,12,971}}]] | ||
|IR Spectra=Olefinic(3002cm^{-1}), cis unsaturation(718cm^{-1}), cis epoxide(835-815cm^{-1})[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]] | |IR Spectra=Olefinic(3002cm^{-1}), cis unsaturation(718cm^{-1}), cis epoxide(835-815cm^{-1})[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]] | ||
| Line 16: | Line 16: | ||
|Biological Activity=Antimicrobial compounds for Piricularia oryzae, a pathogenic fungus of rice blast disease (Imochi-byo)[[Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183|{{RelationTable/GetFirstAuthor|Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183}}]] | |Biological Activity=Antimicrobial compounds for Piricularia oryzae, a pathogenic fungus of rice blast disease (Imochi-byo)[[Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183|{{RelationTable/GetFirstAuthor|Reference:Kato_T:Yamaguthi_H:Uehara_T:Namai_T:,Chemistry and Biology (in Japanese),1986,24,183}}]] | ||
|Note=8, 9-; 10, 11- and 11, 12-Epoxide were detected in autooxidated methyl linoleate as well as 9, 10-epoxide[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]. | |Note=8, 9-; 10, 11- and 11, 12-Epoxide were detected in autooxidated methyl linoleate as well as 9, 10-epoxide[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]]. | ||
}} | |||
{{MassbankSpectra| | |||
UT000226 | |||
UT000227 | |||
UT000228 | |||
UT000229 | |||
UT000230 | |||
UT000231 | |||
UT000232 | |||
UT000233 | |||
UT000234 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8007 |
| LipidMaps | LMFA01070011 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18106EO01 |
| 9,10-EODE | |
|---|---|

| |
| Structural Information | |
| 9,10-Epoxy-12-octadecenoic acid | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
| SMILES | C(C(CC=CCCCCC)1)(CCCCCCCC(O)=O)O1 |
| Physicochemical Information | |
| In autooxidated methyllinoleate Neff_WE et al.. A major secondary product by autooxidation in a lipid film of linoleate Wu_GS et al.. | |
| Antimicrobial compounds for Piricularia oryzae, a pathogenic fungus of rice blast disease (Imochi-byo) Kato_T et al. | |
| 8, 9-; 10, 11- and 11, 12-Epoxide were detected in autooxidated methyl linoleate as well as 9, 10-epoxide Neff_WE et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS Neff_WE et al.: m/e= 310[M], 279[M-OCH3], 199[M-CH3(CH2)4CH=CHCH2], 153[M-(CH2)7-COOCH3], GC-EI-MS(after solvolysation-trimethylsilylation in MeOH) Wu_GS et al. |
| UV Spectra | |
| IR Spectra | Olefinic(3002cm-1), cis unsaturation(718cm-1), cis epoxide(835-815cm-1) Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al.: olefinic protons(5.44ppm), isolated cis epoxide(2.92ppm) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|