LBF18107MO01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01080004 | |LipidMaps=LMFA01080004 | ||
|SysName=13-Hydroxy-9,10-Dimethoxy-11-Octadecenoic Acid | |SysName=13-Hydroxy-9,10-Dimethoxy-11-Octadecenoic Acid | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation) | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508|{{RelationTable/GetFirstAuthor|Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508}}]]: m/e=201[CHOCH3(CH2)7COOCH3](standard peak), 243[M-201] 173[SMTO=CH(CH2)4CH3] | ||
|IR Spectra=Methyl ester(CS2) | |IR Spectra=Methyl ester(CS2)[[Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508|{{RelationTable/GetFirstAuthor|Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508}}]]: trans olefin(973-970cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), OH(3600 and 3440cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>) | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(methyl ester; CDCl3, 300MHz) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(methyl ester; CDCl3, 300MHz)[[Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508|{{RelationTable/GetFirstAuthor|Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508}}]]: C9(3.15-3.19ppm), C10(3.58-3.62ppm), C11(5.55-5.58ppm), C12(5.71ppm), C13(4.15ppm), C9OCH3(3.27-3.29ppm), C10OCH3(3.40-3.41ppm), J11-12=15.7Hz(trans unsaturation) | ||
|NOTE Spectra=ODR analysis | |NOTE Spectra=ODR analysis[[Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508|{{RelationTable/GetFirstAuthor|Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8035 |
| LipidMaps | LMFA01080004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18107MO01 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
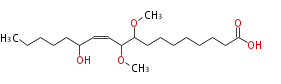
| |
| Structural Information | |
| 13-Hydroxy-9,10-Dimethoxy-11-Octadecenoic Acid | |
| Formula | C20H38O5 |
| Exact Mass | 358.271924326 |
| Average Mass | 358.51272 |
| SMILES | C(CCC(O)C=CC(C(OC)CCCCCCCC(O)=O)OC)CC |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner et al.: m/e=201[CHOCH3(CH2)7COOCH3](standard peak), 243[M-201] 173[SMTO=CH(CH2)4CH3] |
| UV Spectra | |
| IR Spectra | Methyl ester(CS2) Gardner et al.: trans olefin(973-970cm-1), OH(3600 and 3440cm-1) |
| NMR Spectra | 1H-NMR(methyl ester; CDCl3, 300MHz) Gardner et al.: C9(3.15-3.19ppm), C10(3.58-3.62ppm), C11(5.55-5.58ppm), C12(5.71ppm), C13(4.15ppm), C9OCH3(3.27-3.29ppm), C10OCH3(3.40-3.41ppm), J11-12=15.7Hz(trans unsaturation) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|