LBF18107MO01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Other Spectra=ODR analysis[[Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508|{{RelationTable/GetFirstAuthor|Reference:Gardner:HW:Weisleder_D:Nelson_EC:,J. Org. Chem.,1984,49,508}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 21:00, 6 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8035 |
| LipidMaps | LMFA01080004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18107MO01 |
| 13-Hydroxy-9,10-Dimethoxy-11-Octadecenoic Acid | |
|---|---|
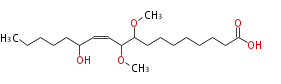
| |
| Structural Information | |
| 13-Hydroxy-9,10-Dimethoxy-11-Octadecenoic Acid | |
| |
| Formula | C20H38O5 |
| Exact Mass | 358.271924326 |
| Average Mass | 358.51272 |
| SMILES | C(CCC(O)C=CC(C(OC)CCCCCCCC(O)=O)OC)CC |
| Physicochemical Information | |
| Oxidative products of 13-hydroperoxylinoleate in MeH Gardner et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner et al.: m/e=201[CHOCH3(CH2)7COOCH3](standard peak), 243[M-201] 173[SMTO=CH(CH2)4CH3] |
| UV Spectra | |
| IR Spectra | Methyl ester(CS2) Gardner et al.: trans olefin(973-970cm-1), OH(3600 and 3440cm-1) |
| NMR Spectra | 1H-NMR(methyl ester; CDCl3, 300MHz) Gardner et al.: C9(3.15-3.19ppm), C10(3.58-3.62ppm), C11(5.55-5.58ppm), C12(5.71ppm), C13(4.15ppm), C9OCH3(3.27-3.29ppm), C10OCH3(3.40-3.41ppm), J11-12=15.7Hz(trans unsaturation) |
| Other Spectra | ODR analysis Gardner et al. |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|