LBF18107PG01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR1821 | |LipidBank=XPR1821 | ||
|LipidMaps=LMFA03010089 | |LipidMaps=LMFA03010089 | ||
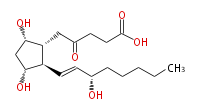
|SysName=5- [ (3R,5S) -Dihydroxy-2R- (3S-hydroxy-1 | |SysName=5- [ (3R,5S) -Dihydroxy-2R- (3S-hydroxy-trans-1-octenyl) cyclopentan-1R-yl ] -4-oxopentanoic acid | ||
|Common Name=&&2,3-dinor-6-keto Prostaglandin F_1 alpha&&5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid&& | |Common Name=&&2,3-dinor-6-keto Prostaglandin F_1 alpha&&5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid&& | ||
|Optical=[ alpha ]^{20.2}_D = +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) <!--1060--> | |Optical=[ alpha ]^{20.2}_D = +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) <!--1060--> | ||
Latest revision as of 06:40, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1821 |
| LipidMaps | LMFA03010089 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18107PG01 |
| 2,3-dinor-6-keto Prostaglandin F1α | |
|---|---|
| |
| Structural Information | |
| 5- [ (3R,5S) -Dihydroxy-2R- (3S-hydroxy-trans-1-octenyl) cyclopentan-1R-yl ] -4-oxopentanoic acid | |
| |
| 2,3-DINOR-6-KETO-PGF1 alpha | |
| Formula | C18H30O6 |
| Exact Mass | 342.204238692 |
| Average Mass | 342.42719999999997 |
| SMILES | [C@@H]([C@@H](CC(=O)CCC(O)=O)1)(C=C[C@H](O)CCCCC)[C@H](O)C[C@@H]1O |
| Physicochemical Information | |
| [ α ]20.2 D = +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) | |
| METHANOL Pace-Asciak_CR et al. | |
| When prostaglandin I2 or its non-enzymatic degradation product (6-keto-prostaglandin F1 alpha ) was infused into man, a major urinary metbolite was 2,3-dinor-6-keto-prostaglandin F1 alpha , a beta -oxidation product Needleman_P et al.. | |
| Spectral Information | |
| Mass Spectra | METHOXIME TRI-TMS ETHER METHYL ESTER ; m/e 601(M+), 586, 570, 530, 511, 496, 480, 440, 421, 390, 350, 300, 294, 263, 217, 205, 191, 73 Pace-Asciak_CR et al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|