LBF18108HO03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA01050127 | |LipidMaps=LMFA01050127 | ||
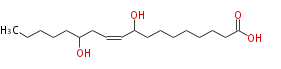
|SysName=9,13-Dihydroxy-10-octadecenoic acid | |SysName=9,13-Dihydroxy-10-octadecenoic acid | ||
|Common Name=&&9,13-Dihydroxy-10- | |Common Name=&&9,13-Dihydroxy-10-octadecenoic acid&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]]: m/e=429[M-43], 355, 285[CH=CH-CH(OTMS) -(CH2)7COOCH3], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027}}]] | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]]: m/e=429[M-43], 355, 285[CH=CH-CH(OTMS) -(CH2)7COOCH3], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1975,39,2027}}]] | ||
|IR Spectra=Trans unsaturation(980cm^{-1}), olefinic(3010cm^{-1}), OH(3620-3500cm^{-1})[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]] | |IR Spectra=Trans unsaturation(980cm^{-1}), olefinic(3010cm^{-1}), OH(3620-3500cm^{-1})[[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]] | ||
Latest revision as of 06:34, 26 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8025 |
| LipidMaps | LMFA01050127 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18108HO03 |
| 9,13-Dihydroxy-10-octadecenoic acid | |
|---|---|
| |
| Structural Information | |
| 9,13-Dihydroxy-10-octadecenoic acid | |
| |
| Formula | C18H34O4 |
| Exact Mass | 314.24570957599997 |
| Average Mass | 314.46016 |
| SMILES | CCCCCC(O)CC=CC(O)CCCCCCCC(O)=O |
| Physicochemical Information | |
| Reaction products between hydroperoxylinoleate and soy bean lipoxygenase Streckert_G et al.. Autooxydation products of methyllinoleate Neff_WE et al. Terao_J et al. Frankel_EN et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) StreckertGet al. Neff_WE et al. TeraoJet al. Frankel_EN et al.: m/e=429[M-43], 355, 285[CH=CH-CH(OTMS) -(CH2)7COOCH3], 259[SMTO=CH-(CH2)7COOCH3], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) StreckertGet al. TeraoJet al. |
| UV Spectra | |
| IR Spectra | Trans unsaturation(980cm-1), olefinic(3010cm-1), OH(3620-3500cm-1) Neff_WE et al. |
| NMR Spectra | 1H-NMR(90MHz,CDCl3): trans olefinic protons(5.6-6.22ppm), carbinol methine protons(4.12ppm), J10-11=12.1Hz(trans unsaturation) Neff_WE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|