LBF18108HO06: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8040 | |LipidBank=DFA8040 | ||
|LipidMaps=LMFA01050137 | |LipidMaps=LMFA01050137 | ||
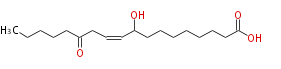
|SysName=9-Hydroxy-13- | |SysName=9-Hydroxy-13-oxo-10-octadecenoic acid | ||
|Common Name=&&9-Hydroxy-13- | |Common Name=&&9-Hydroxy-13-oxo-10-octadecenoic acid&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]][[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]]: m/e=398[M], 383[M-CH3], 367[M-OCH3], 327[M-(CH2)4CH3], 259[SMTO=CH-(CH2)7COOCH3], 241[M-(CH2)7COOCH3], 99[CH3(CH2)4CO] GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]] | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]][[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]]: m/e=398[M], 383[M-CH3], 367[M-OCH3], 327[M-(CH2)4CH3], 259[SMTO=CH-(CH2)7COOCH3], 241[M-(CH2)7COOCH3], 99[CH3(CH2)4CO] GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]] | ||
|IR Spectra=Metyl ester: isolated trans olefin(970cm | |IR Spectra=Metyl ester: isolated trans olefin(970cm^{-1}), keto carbonyl(1717cm^{-1}), OH(3460cm^{-1}) [[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(methyl ester, trans ene)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]: C9(4.08ppm), C10(5.56ppm), C11(5.7ppm), C12(3.11ppm), C2,14(2.33ppm) | ||
|Source=A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[the double bond shows trans configuration][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]] | |Source=A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[the double bond shows trans configuration][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:,Lipids,1979,14,848}}]]. A bitter substance in lecithin[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]. Production mechanism<!--8074-->. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
Latest revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8040 |
| LipidMaps | LMFA01050137 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18108HO06 |
| 9-Hydroxy-13-oxo-10-octadecenoic acid | |
|---|---|
| |
| Structural Information | |
| 9-Hydroxy-13-oxo-10-octadecenoic acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC(=O)CC=CC(O)CCCCCCCC(O)=O |
| Physicochemical Information | |
| A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[the double bond shows trans configuration] Gardner_HW et al. Gardner_HW et al.. A bitter substance in lecithin Sessa_DJ et al.. Production mechanism. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner_HW et al. Sessa_DJ et al. Gardner_HW et al.: m/e=398[M], 383[M-CH3], 367[M-OCH3], 327[M-(CH2)4CH3], 259[SMTO=CH-(CH2)7COOCH3], 241[M-(CH2)7COOCH3], 99[CH3(CH2)4CO] GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) Gardner_HW et al. |
| UV Spectra | |
| IR Spectra | Metyl ester: isolated trans olefin(970cm-1), keto carbonyl(1717cm-1), OH(3460cm-1) Gardner_HW et al. Sessa_DJ et al. |
| NMR Spectra | 1H-NMR(methyl ester, trans ene) Gardner_HW et al.: C9(4.08ppm), C10(5.56ppm), C11(5.7ppm), C12(3.11ppm), C2,14(2.33ppm) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|