LBF18109AM01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR7014 | |LipidBank=XPR7014 | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName= | |SysName=Octadecenoic acidamide | ||
|Common Name=&&Oleoylamide&& | |Common Name=&&Oleoylamide&&Octadecenamide&& | ||
|Melting Point=74-75°C[[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Melting Point=74-75°C[[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|NMR Spectra=^1 H NMR (CDCl3) delta 5.42 (br s, 2H), 5.32-5.36 (m, 2H), 2.22 (t, J=7.8 Hz 2H), 1.98-2.02 (m,6H), 1.62-1.66 (m, 4H), 1.27-1.31 (m, 16H), 0.88 (t, J=6.9Hz, 3H). [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |NMR Spectra=^1 H NMR (CDCl3) delta 5.42 (br s, 2H), 5.32-5.36 (m, 2H), 2.22 (t, J=7.8 Hz 2H), 1.98-2.02 (m,6H), 1.62-1.66 (m, 4H), 1.27-1.31 (m, 16H), 0.88 (t, J=6.9Hz, 3H). [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
Revision as of 07:06, 26 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7014 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18109AM01 |
| Oleoylamide | |
|---|---|
| |
| Structural Information | |
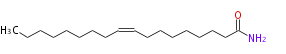
| Octadecenoic acidamide | |
| |
| Formula | C18H35NO |
| Exact Mass | 281.271864747 |
| Average Mass | 281.47664 |
| SMILES | CCCCCCCCC=CCCCCCCCC(N)=O |
| Physicochemical Information | |
| 74-75°C Sheskin_T et al. | |
| This compound was synthesized from oleic acid and ammonium hydroxide in 60 % yield. Sheskin_T et al. | |
| Binding of this compound to the brain cannabinoid receptor (CBl)was not found. Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) δ 5.42 (br s, 2H), 5.32-5.36 (m, 2H), 2.22 (t, J=7.8 Hz 2H), 1.98-2.02 (m,6H), 1.62-1.66 (m, 4H), 1.27-1.31 (m, 16H), 0.88 (t, J=6.9Hz, 3H). SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|