LBF18206HP04: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8005 | |LipidBank=DFA8005 | ||
|LipidMaps=LMFA01040008 | |LipidMaps=LMFA01040008 | ||
|SysName=8-Hydroperoxy-9,12- | |SysName=8-Hydroperoxy-9,12-octadecadienoic acid | ||
|Common Name=&&8-Hydroperoxy-9,12- | |Common Name=&&8-Hydroperoxy-9,12-octadecadienoic acid&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis, reduction and trimethylsilylation) [[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]: m/e= 292[M-HOTMS], 271[CH=CH-CH(OTMS)-(CH2)6COOCH3], 239[M-(CH2)6C00CH3], 173[SMTO=CH-(CH2)6COOCH3-TMS+H], 149[239-HOTMS] standard peak | |Mass Spectra=GC-EI-MS(after methanolysis, reduction and trimethylsilylation) [[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]: m/e= 292[M-HOTMS], 271[CH=CH-CH(OTMS)-(CH2)6COOCH3], 239[M-(CH2)6C00CH3], 173[SMTO=CH-(CH2)6COOCH3-TMS+H], 149[239-HOTMS] standard peak | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR (after methanolyzation, reduction and 400MHz )[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]: olefinic protons(5.91-5.51ppm), C8 (4.45ppm), C11(2.84ppm), C14(2.05ppm),J9-10= J12-13= 10.7Å }0.2Hz (cis) | ||
|Source=A minor component of monohydroperoxide generated from linoleic acid or methyllinoleate by autooxidation[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]<!--8051-->. A minor component of hydroperoxide generated from linoleic acid through oxidation by soy bean lipoxygenase (type-2)[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]. | |Source=A minor component of monohydroperoxide generated from linoleic acid or methyllinoleate by autooxidation[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]<!--8051-->. A minor component of hydroperoxide generated from linoleic acid through oxidation by soy bean lipoxygenase (type-2)[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Note=8-OOH and 14-OOH are known as small amount components of monohydroperoxidate from auto oxidation of linoleic acid and oxidation of soybean lipoxygenase(type-2). Their amount does not change by temperature or addition of | |Note=8-OOH and 14-OOH are known as small amount components of monohydroperoxidate from auto oxidation of linoleic acid and oxidation of soybean lipoxygenase(type-2). Their amount does not change by temperature or addition of α -tocopherol[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8005 |
| LipidMaps | LMFA01040008 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206HP04 |
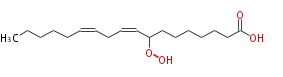
| 8-Hydroperoxy-9,12-octadecadienoic acid | |
|---|---|
| |
| Structural Information | |
| 8-Hydroperoxy-9,12-octadecadienoic acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC=CCC=CC(OO)CCCCCCC(O)=O |
| Physicochemical Information | |
| A minor component of monohydroperoxide generated from linoleic acid or methyllinoleate by autooxidation Haslbeck_F et al.. A minor component of hydroperoxide generated from linoleic acid through oxidation by soy bean lipoxygenase (type-2) Haslbeck_F et al.. | |
| 8-OOH and 14-OOH are known as small amount components of monohydroperoxidate from auto oxidation of linoleic acid and oxidation of soybean lipoxygenase(type-2). Their amount does not change by temperature or addition of α -tocopherol Haslbeck_F et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis, reduction and trimethylsilylation) HaslbeckFet al.: m/e= 292[M-HOTMS], 271[CH=CH-CH(OTMS)-(CH2)6COOCH3], 239[M-(CH2)6C00CH3], 173[SMTO=CH-(CH2)6COOCH3-TMS+H], 149[239-HOTMS] standard peak |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H-NMR (after methanolyzation, reduction and 400MHz ) HaslbeckFet al.: olefinic protons(5.91-5.51ppm), C8 (4.45ppm), C11(2.84ppm), C14(2.05ppm),J9-10= J12-13= 10.7Å }0.2Hz (cis) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|