LBF18206HP05: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01040009 | |LipidMaps=LMFA01040009 | ||
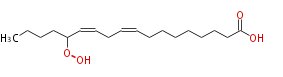
|SysName=14-Hydroperoxy-9,12-Octadecadienoic Acid | |SysName=14-Hydroperoxy-9,12-Octadecadienoic Acid | ||
|Mass Spectra=GC-EI-MS(after methanolysis, reduction and trimethylsilylation) | |Mass Spectra=GC-EI-MS(after methanolysis, reduction and trimethylsilylation)[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]: m/e= 325[M-(CH2)3CH3] standard peak, 292[M-HOTMS], 235[325-HOTMS], 185[CH=CH-CH(OTMS)-(CH2)3CH3] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(after methanolyzation, reduction and 400MHz) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(after methanolyzation, reduction and 400MHz)[[Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185|{{RelationTable/GetFirstAuthor|Reference:Haslbeck_F:Grosch_W:Firl_J:,Biochim. Biophys. Acta,1983,750,185}}]]: olefinic protons(5.32-5.47ppm) C14(4.45ppm), C11(2.85ppm), C8(2.05ppm), J9-10= J12-13= 10.08Å }0.1Hz(cis) | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8006 |
| LipidMaps | LMFA01040009 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18206HP05 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
| 14-Hydroperoxy-9,12-Octadecadienoic Acid | |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCC(OO)C=CCC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis, reduction and trimethylsilylation) HaslbeckFet al.: m/e= 325[M-(CH2)3CH3] standard peak, 292[M-HOTMS], 235[325-HOTMS], 185[CH=CH-CH(OTMS)-(CH2)3CH3] |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H-NMR(after methanolyzation, reduction and 400MHz) HaslbeckFet al.: olefinic protons(5.32-5.47ppm) C14(4.45ppm), C11(2.85ppm), C8(2.05ppm), J9-10= J12-13= 10.08Å }0.1Hz(cis) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|