LBF18207HO04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01050131 | |LipidMaps=LMFA01050131 | ||
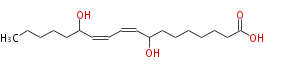
|SysName=8,13-Dihydroxy-9,11-Octadecadienoic Acid | |SysName=8,13-Dihydroxy-9,11-Octadecadienoic Acid | ||
|Common Name=&&8,13-Dihydroxy-9,11-Octadecadienoic Acid&& | |||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]]: m/e=455[M-CH3], 439[M-OCH3], 380[M-HOTMS], 245[SMTO=CH-(CH2)6COOCH3], 237[M-(CH2)6COOCH3-HOTMS], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]]: m/e=459[M-CH3], 403[M-(CH2)4CH3], 374[(CH2)4 -CH(OTMS)-(CH2)6-C(OTMS)OCH] | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]]: m/e=455[M-CH3], 439[M-OCH3], 380[M-HOTMS], 245[SMTO=CH-(CH2)6COOCH3], 237[M-(CH2)6COOCH3-HOTMS], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]]: m/e=459[M-CH3], 403[M-(CH2)4CH3], 374[(CH2)4 -CH(OTMS)-(CH2)6-C(OTMS)OCH] | ||
|UV Spectra=Methyl ester: <FONT FACE="Symbol">l</FONT>MeOH/max=230nm(224nm and 238nm)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]][[Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353}}]] | |UV Spectra=Methyl ester: <FONT FACE="Symbol">l</FONT>MeOH/max=230nm(224nm and 238nm)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]][[Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353}}]] | ||
|IR Spectra=trans trans unsaturation(990-981cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]][[Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353}}]] | |IR Spectra=trans trans unsaturation(990-981cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>)[[Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta,1983,752,191}}]][[Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Biochim. Biophys. Acta - Lipids and Lipid Metabolism,1983,752,353}}]] | ||
}} | }} | ||
Revision as of 00:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8029 |
| LipidMaps | LMFA01050131 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HO04 |
| 8,13-Dihydroxy-9,11-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
| 8,13-Dihydroxy-9,11-Octadecadienoic Acid | |
| |
| Formula | C18H32O4 |
| Exact Mass | 312.23005951199997 |
| Average Mass | 312.44428 |
| SMILES | CCCCCC(O)C=CC=CC(O)CCCCCCC(O)=O |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) HambergM: m/e=455[M-CH3], 439[M-OCH3], 380[M-HOTMS], 245[SMTO=CH-(CH2)6COOCH3], 237[M-(CH2)6COOCH3-HOTMS], 173[SMTO=CH-(CH2)4CH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) HambergM: m/e=459[M-CH3], 403[M-(CH2)4CH3], 374[(CH2)4 -CH(OTMS)-(CH2)6-C(OTMS)OCH] |
| UV Spectra | Methyl ester: lMeOH/max=230nm(224nm and 238nm) HambergM HambergM |
| IR Spectra | trans trans unsaturation(990-981cm-1) HambergM HambergM |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|