LBF18207HP03
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8057 |
| LipidMaps | LMFA01040041 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HP03 |
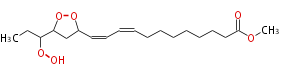
| Methyl-13,15-epidioxy-16-hydroperoxy-9,11-octadecadienoic acid | |
|---|---|
| |
| Structural Information | |
| Methyl-13,15-epidioxy-16-hydroperoxy-9,11-octadecadienoic acid | |
| |
| Formula | C19H32O6 |
| Exact Mass | 356.219888756 |
| Average Mass | 356.45378 |
| SMILES | C(O1)(CC(C=CC=CCCCCCCCC(=O)OC)O1)C(CC)OO |
| Physicochemical Information | |
| Auto oxydation of linoleate Neff_WE et al. Coxon_DT et al.. Oxydation of linoleate in the presence of Fe(III)-ascorbic acid Toyoda_I et al.. Photoenhancemant of linoleate peroxydation[Type II] Neff_WE et al. Neff_WE et al.. Production mechanism Frankel_EN Frankel_EN . | |
| It reacts with DNA in the presence of Fe ions and ascorbic acid Fujimoto_K et al.. | |
| Major secondary products of linolenate by autooxidation Frankel_EN Neff_WE et al.. It is formed through 1, 3 cyclization of 13-peroxy radical of linolenate Frankel_EN Frankel_EN Frankel_EN Chan_HWS et al. Coxon_DT et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction(PH3P) and TMS-derivatization) Neff_WE et al.: m/e=397[M-CH3], 131[SMTO=CHCH2CH3]; GC-EI-MS(after reduction(NaBH4 or KI) and TMS-derivatization) Frankel_EN et al.: m/e=311[SMTO=CH-CH=CH-CH=CH-(CH2)7COOCH3]; 131[SMTO=CHCH2CH3] |
| UV Spectra | Conjugated cis, trans diene: λ max=234-237nm, conjugated trans, trans diene: λ max=231-234nm ToyodaIet al. Neff_WE et al. Coxon_DT et al. Neff_WE et al. Chan_HWS et al. |
| IR Spectra | OOH group: 3720-3140cm-1[bonded], 3530-3520cm-1[FREE]; olefinic protons: 3020-3000cm-1; conjugated cis, trans diene : 989-980cm-1, 955-947cm-1; conjugatedtrans, trans diene: 992-984cm-1, 955cm-1 ToyodaIet al. Neff_WE et al. Coxon_DT et al. Neff_WE et al. Neff_WE et al. Chan_HWS et al. |
| NMR Spectra | 1H-NMR Neff_WE et al. Coxon_DT et al. Neff_WE et al. Chan_HWS et al.: C9: 5.46-5.78; C10: 5.99-6.05; C11: 6.26-6.67; C12: 5.53- 5.62; C13: 4.75-4.84; C14: 2.23-2.47, 2.79-2.88; C15: 4.47-4.49; C16: 3.86-4.15; OOH: 8.98-9.55ppm; J9-15=10.0-11.0[cis]; J9-10=15.1-15.5[trans]; J11-12=14.5-15.4Hz[trans] |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|