LBF18207OX01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&13-Oxo-9,11-Octadecadienoic Acid&&13-Oxo-9,11-Octadecadienoate&& | |Common Name=&&13-Oxo-9,11-Octadecadienoic Acid&&13-Oxo-9,11-Octadecadienoate&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87}}]]: m/e=308[M], 277[M-OCH3], 252[M-CH2=C H-CH2CH3], 237[M-(CH2)4CH3], 209[M-C(O)(CH2)4CH3], 151[M-(CH2)7COOCH3], GC-EI-MS(TMS)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]: m/e=366[M], 341[M-CH3], 295[M-(CH2)4CH3], 276[M-HOTMS], 166[M-(CH2)6C(=O)OTMS]; REARRA | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Nelson_EC:Tjarks_LW:England_RE:,Chem. Phys. Lipids,1984,35,87}}]]: m/e=308[M], 277[M-OCH3], 252[M-CH2=C H-CH2CH3], 237[M-(CH2)4CH3], 209[M-C(O)(CH2)4CH3], 151[M-(CH2)7COOCH3], GC-EI-MS(TMS)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]: m/e=366[M], 341[M-CH3], 295[M-(CH2)4CH3], 276[M-HOTMS], 166[M-(CH2)6C(=O)OTMS]; REARRA | ||
|UV Spectra= | |UV Spectra=lambdaMeOH/max=277-278nm(epsilon=20300)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]](038/078/075), lambda EtOH /max=278nm[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]], lambdamax=267nm(cyclohexane)[[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]], DNP hydrazone: lambdaCHCl3/max=388, 304, 265nm[[Reference:Garssen_GJ:Vliegenthart_JF:Boldingh_J:,Biochem. J.,1971,122,327|{{RelationTable/GetFirstAuthor|Reference:Garssen_GJ:Vliegenthart_JF:Boldingh_J:,Biochem. J.,1971,122,327}}]] | ||
|IR Spectra=Trans, trans unsaturations(strong absorption at 1000-990cm | |IR Spectra=Trans, trans unsaturations(strong absorption at 1000-990cm^- ^1 ), cis, trans unsaturations(960-955cm^- ^1 ), unsaturated ketone(1695-1600cm^- ^1 )[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448|{{RelationTable/GetFirstAuthor|Reference:Schieberle_P:Tsoukalas_B:Grosch_W:,Z. Lebensm. Unters. Forsch.,1979,168,448}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]]: C8(2.20-2.23ppm), C9, 10, 12(6.06-6.21ppm), C11(7.02-7.51ppm) C14(2.54ppm) | ||
|Source=A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[trans, trans; or trans, cis][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II)[[Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177|{{RelationTable/GetFirstAuthor|Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177}}]]. | |Source=A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[trans, trans; or trans, cis][[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II)[[Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177|{{RelationTable/GetFirstAuthor|Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8047 |
| LipidMaps | LMFA01060072 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207OX01 |
| 13-Oxo-9,11-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
| 13-Oxo-9,11-Octadecadienoic Acid/13-Oxo-9,11-Octadecadienoate | |
| |
| Formula | C18H30O3 |
| Exact Mass | 294.21949482599996 |
| Average Mass | 294.429 |
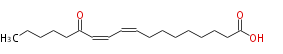
| SMILES | CCCCCC(=O)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| A degradation product of hydroperoxylinoleate in the presence of Fe(III)-cystein[trans, trans; or trans, cis] Gardner_HW et al.. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II) Yamamoto_Y et al.. | |
| It showed a slightly lower toxicity than linoleate monohydroxyperoxide Fujimoto_K . | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) HambergM Sessa_DJ et al. Frankel_EN et al. Gardner_HW et al.: m/e=308[M], 277[M-OCH3], 252[M-CH2=C H-CH2CH3], 237[M-(CH2)4CH3], 209[M-C(O)(CH2)4CH3], 151[M-(CH2)7COOCH3], GC-EI-MS(TMS) Gardner_HW et al.: m/e=366[M], 341[M-CH3], 295[M-(CH2)4CH3], 276[M-HOTMS], 166[M-(CH2)6C(=O)OTMS]; REARRA |
| UV Spectra | lambdaMeOH/max=277-278nm(ε=20300) Gardner_HW et al. StreckertGet al. SchieberlePet al.(038/078/075), λ EtOH /max=278nm Gardner_HW et al., lambdamax=267nm(cyclohexane) Sessa_DJ et al., DNP hydrazone: lambdaCHCl3/max=388, 304, 265nm Garssen_GJ et al. |
| IR Spectra | Trans, trans unsaturations(strong absorption at 1000-990cm-1), cis, trans unsaturations(960-955cm-1), unsaturated ketone(1695-1600cm-1) Gardner_HW et al. StreckertGet al. SchieberlePet al. Sessa_DJ et al. |
| NMR Spectra | 1H-NMR Gardner_HW et al. Sessa_DJ et al.: C8(2.20-2.23ppm), C9, 10, 12(6.06-6.21ppm), C11(7.02-7.51ppm) C14(2.54ppm) |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|