LBF18302HP01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&Methyl-15-Hydroperoxy-9,12,16-Octadecatrienoate&& | |Common Name=&&Methyl-15-Hydroperoxy-9,12,16-Octadecatrienoate&& | ||
|Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]]: m/e=271[O=CH(CH2)13C(=OH)OCH3]; 242[CH2(CH2)12C(=OH)OCH3]; 239[O=CH(CH2)13C=O]; GC-EI-MS(Me-ester; after reduction and hydroganation)[[Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234}}]]: m/e=143[SMTO=CH-CH=CH-CH3] | |Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]]: m/e=271[O=CH(CH2)13C(=OH)OCH3]; 242[CH2(CH2)12C(=OH)OCH3]; 239[O=CH(CH2)13C=O]; GC-EI-MS(Me-ester; after reduction and hydroganation)[[Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234}}]]: m/e=143[SMTO=CH-CH=CH-CH3] | ||
|Source=Oxidation of linoleate by singlet oxygen[[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]];>. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8055 |
| LipidMaps | LMFA01040039 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18302HP01 |
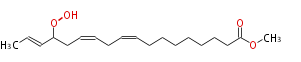
| Methyl-15-Hydroperoxy-9,12,16-Octadecatrienoate | |
|---|---|
| |
| Structural Information | |
| Methyl-15-Hydroperoxy-9,12,16-Octadecatrienoate | |
| |
| Formula | C19H32O4 |
| Exact Mass | 324.23005951199997 |
| Average Mass | 324.45498 |
| SMILES | CC=CC(OO)CC=CCC=CCCCCCCCC(=O)OC |
| Physicochemical Information | |
| Oxidation of linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN ;>. | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction and hydrogenation) Chan_HWS : m/e=271[O=CH(CH2)13C(=OH)OCH3]; 242[CH2(CH2)12C(=OH)OCH3]; 239[O=CH(CH2)13C=O]; GC-EI-MS(Me-ester; after reduction and hydroganation) TeraoJet al.: m/e=143[SMTO=CH-CH=CH-CH3] |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|