LBF18307HP01: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8151 | |LipidBank=DFA8151 | ||
|LipidMaps=LMFA01040034 | |LipidMaps=LMFA01040034 | ||
|SysName=13S- | |SysName=13S-Hydroperoxy-(cis-6,cis-9,trans-11)-octadecatrienoic acid | ||
|Common Name=&&13S- | |Common Name=&&13S-Hydroperoxy- (6Z,9Z,11E) -octadecatrienoic acid&&13-HpOTrE(r)&& | ||
|UV Spectra= lambda max: 235nm epsilon : 23,000 | |UV Spectra= lambda max: 235nm epsilon : 23,000 | ||
|Source=13(S)-HpOTrE( gamma ) is produced from gamma -linolenic acid by soybean 15-lipoxygenase [[Reference:Funk_MO:Isacc_R:Porter_NA:,Lipids,1976,11,113|{{RelationTable/GetFirstAuthor|Reference:Funk_MO:Isacc_R:Porter_NA:,Lipids,1976,11,113}}]]. | |Source=13(S)-HpOTrE( γ ) is produced from γ -linolenic acid by soybean 15-lipoxygenase [[Reference:Funk_MO:Isacc_R:Porter_NA:,Lipids,1976,11,113|{{RelationTable/GetFirstAuthor|Reference:Funk_MO:Isacc_R:Porter_NA:,Lipids,1976,11,113}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Symbol=13(S)-HpOTrE( gamma ) | |Symbol=13(S)-HpOTrE( γ ) | ||
}} | |||
{{MassbankSpectra| | |||
UT000073 | |||
UT000074 | |||
UT000075 | |||
UT000076 | |||
UT000077 | |||
UT000078 | |||
UT000079 | |||
UT000080 | |||
UT000081 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8151 |
| LipidMaps | LMFA01040034 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18307HP01 |
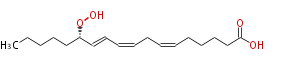
| 13S-Hydroperoxy- (6Z,9Z,11E) -octadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 13S-Hydroperoxy-(cis-6,cis-9,trans-11)-octadecatrienoic acid | |
| |
| 13(S)-HpOTrE( γ ) | |
| Formula | C18H30O4 |
| Exact Mass | 310.21440944799997 |
| Average Mass | 310.4284 |
| SMILES | CCCCCC(OO)C=CC=CCC=CCCCCC(O)=O |
| Physicochemical Information | |
| 13(S)-HpOTrE( γ ) is produced from γ -linolenic acid by soybean 15-lipoxygenase Funk_MO et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 235nm ε : 23,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|








