LBF20107PG02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
|IR Spectra=KBr: <FONT FACE="Symbol">n</FONT> 3400, 1740, 1720, 1710, 1245, 1160, 1075, 970 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |IR Spectra=KBr: <FONT FACE="Symbol">n</FONT> 3400, 1740, 1720, 1710, 1245, 1160, 1075, 970 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 5.58(m, 2H, 13,14-CH), 4.09(m, 1H, 11-CH), 4.02(m, 1H, 15-CH), 2.7(2H, 7-CH), 2.69(IH, 10<FONT FACE="Symbol">b</FONT>-CH), 2.45-2.47(m, 3H, 5,8,12-CH), 2.29(1H, 10<FONT FACE="Symbol">a</FONT>-CH), 2.28(2H, 2-CH), 1.58 - 1.32(12H), 0.91(t, 3H, 20-CH<SUB><FONT SIZE=-1>3</FONT></SUB>) [[Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1982,60,2617|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1982,60,2617}}]]. METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>C-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : 216.6, 208.4, 173.8, 138.0, 126.5, 72.3, 51.5, 44.6, 45.7, 42.4, 37.6, 33.8, 31.8, 25.2, 24.5, 23.4, 22.6, 14.0 [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 5.58(m, 2H, 13,14-CH), 4.09(m, 1H, 11-CH), 4.02(m, 1H, 15-CH), 2.7(2H, 7-CH), 2.69(IH, 10<FONT FACE="Symbol">b</FONT>-CH), 2.45-2.47(m, 3H, 5,8,12-CH), 2.29(1H, 10<FONT FACE="Symbol">a</FONT>-CH), 2.28(2H, 2-CH), 1.58 - 1.32(12H), 0.91(t, 3H, 20-CH<SUB><FONT SIZE=-1>3</FONT></SUB>) [[Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1982,60,2617|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:,Can. J. Chem.,1982,60,2617}}]]. METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>C-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : 216.6, 208.4, 173.8, 138.0, 126.5, 72.3, 51.5, 44.6, 45.7, 42.4, 37.6, 33.8, 31.8, 25.2, 24.5, 23.4, 22.6, 14.0 [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|Source=There were reports of prolonged biological activity of chemically unstable prostaglandin I2, which suggested a possible transformation of prostaglandin I2 to a more stable metabolite with potent bioactivity [[Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021|{{RelationTable/GetFirstAuthor|Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021}}]] | |Source=There were reports of prolonged biological activity of chemically unstable prostaglandin I2, which suggested a possible transformation of prostaglandin I2 to a more stable metabolite with potent bioactivity [[Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021|{{RelationTable/GetFirstAuthor|Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021}}]]. Futher investigations led to the discovery of 6-keto-prostaglandin E1. | ||
|Chemical Synthesis=[[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |Chemical Synthesis=[[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] {{Image200|LBF20107PG02FT0001.gif}} | ||
|Metabolism=A potential role of 9-hydroxyprostaglandin dehydrogenase was demonstrated in the transformation of prostaglandin I2 to 6-keto-prostaglandin E1 [[Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021|{{RelationTable/GetFirstAuthor|Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021}}]] | |Metabolism=A potential role of 9-hydroxyprostaglandin dehydrogenase was demonstrated in the transformation of prostaglandin I2 to 6-keto-prostaglandin E1 [[Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021|{{RelationTable/GetFirstAuthor|Reference:Wong_PY:Lee_WH:Chao_PH:Reiss_RF:McGiff_JC:,J. Biol. Chem.,1980,255,9021}}]]. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 11:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1430 |
| LipidMaps | LMFA03010012 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG02 |
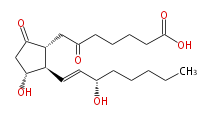
| 6-KETO-PROSTAGLANDIN E1 | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -5-oxocyclopentan-1 (R) -yl ] -6-oxoheptanoic acid | |
| |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@@H](CC(=O)CCCCC(O)=O)1)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 65°C Tanaka_T et al. | |
| METHANOL, CHLOROFORM TanakaTet al. | |
| There were reports of prolonged biological activity of chemically unstable prostaglandin I2, which suggested a possible transformation of prostaglandin I2 to a more stable metabolite with potent bioactivity Wong_PY et al.. Futher investigations led to the discovery of 6-keto-prostaglandin E1. | |
Tanaka_T et al.  | |
| A potential role of 9-hydroxyprostaglandin dehydrogenase was demonstrated in the transformation of prostaglandin I2 to 6-keto-prostaglandin E1 Wong_PY et al.. | |
| Spectral Information | |
| Mass Spectra | DIRECT EXPOSURE AMMONIA CI, POSITIVE : 386(M++18), 368(M+), 351, 350, 244, 136. NEGATIVE : 368(M+) ,350, 338, 332 Cepa_SR et al. |
| UV Spectra | |
| IR Spectra | KBr: n 3400, 1740, 1720, 1710, 1245, 1160, 1075, 970 cm-1 TanakaTet al. |
| NMR Spectra | 1H-NMR(CDCl3) : d 5.58(m, 2H, 13,14-CH), 4.09(m, 1H, 11-CH), 4.02(m, 1H, 15-CH), 2.7(2H, 7-CH), 2.69(IH, 10b-CH), 2.45-2.47(m, 3H, 5,8,12-CH), 2.29(1H, 10a-CH), 2.28(2H, 2-CH), 1.58 - 1.32(12H), 0.91(t, 3H, 20-CH3) KotovychGet al.. METHYL ESTER ; 13C-NMR(CDCl3) : 216.6, 208.4, 173.8, 138.0, 126.5, 72.3, 51.5, 44.6, 45.7, 42.4, 37.6, 33.8, 31.8, 25.2, 24.5, 23.4, 22.6, 14.0 TanakaTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|