LBF20107PG05: Difference between revisions
No edit summary |
No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1710 | |LipidBank=XPR1710 | ||
|LipidMaps=LMFA03010147 | |LipidMaps=LMFA03010147 | ||
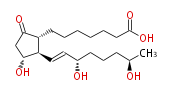
|SysName=9- | |SysName=9-Oxo-11 (11alpha,15S,19R) -trihydroxy-prost-trans-13-en-1-oic acid | ||
|Common Name=&& | |Common Name=&&19R-hydroxy Prostaglandin E_1&&9-Oxo- (11alpha,15S,19R) -trihydroxy-prost-13(E)-en-1-oic acid&& | ||
|Source=19(R)-hydroxy PGE1 is the major prostaglandin found in the semen of primates. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Biological Activity= 19(R)-hydroxy PGE1 has contractile activity on smooth muscle preparations.[[Reference:Kelly_RW:Taylor_PL:Hearn_JP:Short_RV:Martin_DE:Marston_JH:,Nature,1976,260,544|{{RelationTable/GetFirstAuthor|Reference:Kelly_RW:Taylor_PL:Hearn_JP:Short_RV:Martin_DE:Marston_JH:,Nature,1976,260,544}}]][[Reference:Woodward_DF:Protzman_CE:Krauss_AH:Williams_LS:,Prostaglandins,1993,46,371|{{RelationTable/GetFirstAuthor|Reference:Woodward_DF:Protzman_CE:Krauss_AH:Williams_LS:,Prostaglandins,1993,46,371}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:01, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1710 |
| LipidMaps | LMFA03010147 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG05 |
| 19R-hydroxy Prostaglandin E1 | |
|---|---|
| |
| Structural Information | |
| 9-Oxo-11 (11α,15S,19R) -trihydroxy-prost-trans-13-en-1-oic acid | |
| |
| Formula | C20H34O6 |
| Exact Mass | 370.23553882 |
| Average Mass | 370.48036 |
| SMILES | [C@H]([C@@H](C=C[C@H](O)CCC[C@@H](C)O)1)(C(C[C@H]1O)=O)CCCCCCC(O)=O |
| Physicochemical Information | |
| 19(R)-hydroxy PGE1 is the major prostaglandin found in the semen of primates. | |
| 19(R)-hydroxy PGE1 has contractile activity on smooth muscle preparations. Kelly_RW et al. Woodward_DF et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|