LBF20107PG18: Difference between revisions
No edit summary |
No edit summary |
||
| (13 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR1811 | |LipidBank=XPR1811 | ||
|LipidMaps=LMFA03010001 | |LipidMaps=LMFA03010001 | ||
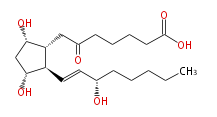
|SysName=7- [ | |SysName=7- [ (3R,5S) -Dihydroxy- 2R- (3S-hydroxy-trans-1-octenyl) cyclopentan-1R -yl ] -6-oxoheptanoic acid | ||
|Common Name=&&6- | |Common Name=&&6-keto Prostaglandin F_1alpha&&7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -6-oxoheptanoic acid && | ||
| | |Optical=[ alpha ]^{21}_D = -9.6° (C=1.04 METHANOL) [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|Solubility=DIETHYL ETHER[[Reference:Pace-Asciak_C:,J. Am. Chem. Soc.,1976,98,2348|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_C:,J. Am. Chem. Soc.,1976,98,2348}}]]METHANOL, ACETONE, ETHYL ACETATE [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |Solubility=DIETHYL ETHER[[Reference:Pace-Asciak_C:,J. Am. Chem. Soc.,1976,98,2348|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_C:,J. Am. Chem. Soc.,1976,98,2348}}]]METHANOL, ACETONE, ETHYL ACETATE [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|Mass Spectra=METHYL ESTER ; 366(M | |Mass Spectra=METHYL ESTER ; 366(M^+ -18), 348, 335, 330, 323, 319, 279, 265, 223, 196, 195, 164, 143, 121, 111, 99, 95, 71 [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]]. DIRECT EXPOSURE AMMONIA CI POSITIVE : 370, 353, 244, 163, 153, 136. NEGATIVE : 368, 351, 334, 316, 225, 219, 166, 135, 127 [[Reference:Cepa_SR:Hall_ER:Venton_DL:,Prostaglandins,1984,27,645|{{RelationTable/GetFirstAuthor|Reference:Cepa_SR:Hall_ER:Venton_DL:,Prostaglandins,1984,27,645}}]] | ||
|IR Spectra=NEAT: | |IR Spectra=NEAT: nu 3400, 1715, 1245, 1045, 975, 915, 875, 845, 800, 730 cm^{-1} [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(ACETONE-D_6 ) : delta 6.1-5.4(bs, 4H), 5.5-5.2(m, 2H), 4.7-3.5(m, 3H), 2.5-1.1 (m, 22H), 0.86(t, 3H) [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|Source=When prostaglandin I2 is produced in animal tissues, it is unstable in aqueous solution, especially at acidic pH, and readily decomposed to 6-keto-prostaglandin F1 | |Source=When prostaglandin I2 is produced in animal tissues, it is unstable in aqueous solution, especially at acidic pH, and readily decomposed to 6-keto-prostaglandin F1 alpha [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]]. Therefore, 6-keto-prostaglandin F1 alpha is detected where prostaglandin I2 is produced. | ||
|Chemical Synthesis=[[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |Chemical Synthesis=[[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] {{Image200|LBF20107PG18FT0001.gif}} | ||
|Metabolism=6-Keto-prostaglandin F1 | |Metabolism=6-Keto-prostaglandin F1 alpha is subjected to beta -oxidation, and converted to 2,3-dinor-6-keto-prostaglandin F1 alpha which appears in urine as a major metabolite [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]]. | ||
|Symbol=6-KETO-PGF1α | |||
|Biological Activity=Degradation of prostaglandin I2 to 6-keto-prostaglandin F1 alpha brings about the loss of biological activities. For example, the hypotensive effect of prostaglandin I2 is at least 100 times mor active than 6-keto-prostaglandin F1 alpha [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 08:14, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1811 |
| LipidMaps | LMFA03010001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG18 |
| 6-keto Prostaglandin F_1α | |
|---|---|
| |
| Structural Information | |
| 7- [ (3R,5S) -Dihydroxy- 2R- (3S-hydroxy-trans-1-octenyl) cyclopentan-1R -yl ] -6-oxoheptanoic acid | |
| |
| 6-KETO-PGF1α | |
| Formula | C20H34O6 |
| Exact Mass | 370.23553882 |
| Average Mass | 370.48036 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@@H](CC(=O)CCCCC(O)=O)1)[C@@H](C[C@@H]1O)O)CC |
| Physicochemical Information | |
| [ α ]21 D = -9.6° (C=1.04 METHANOL) TanakaTet al. | |
| DIETHYL ETHER Pace-AsciakCMETHANOL, ACETONE, ETHYL ACETATE TanakaTet al. | |
| When prostaglandin I2 is produced in animal tissues, it is unstable in aqueous solution, especially at acidic pH, and readily decomposed to 6-keto-prostaglandin F1 alpha Moncada_S et al.. Therefore, 6-keto-prostaglandin F1 alpha is detected where prostaglandin I2 is produced. | |
|
Tanaka_T et al. | |
| 6-Keto-prostaglandin F1 alpha is subjected to beta -oxidation, and converted to 2,3-dinor-6-keto-prostaglandin F1 alpha which appears in urine as a major metabolite Needleman_P et al.. | |
| Degradation of prostaglandin I2 to 6-keto-prostaglandin F1 alpha brings about the loss of biological activities. For example, the hypotensive effect of prostaglandin I2 is at least 100 times mor active than 6-keto-prostaglandin F1 alpha Moncada_S et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; 366(M+-18), 348, 335, 330, 323, 319, 279, 265, 223, 196, 195, 164, 143, 121, 111, 99, 95, 71 TanakaTet al.. DIRECT EXPOSURE AMMONIA CI POSITIVE : 370, 353, 244, 163, 153, 136. NEGATIVE : 368, 351, 334, 316, 225, 219, 166, 135, 127 Cepa_SR et al. |
| UV Spectra | |
| IR Spectra | NEAT: ν 3400, 1715, 1245, 1045, 975, 915, 875, 845, 800, 730 cm-1 TanakaTet al. |
| NMR Spectra | 1H-NMR(ACETONE-D6) : δ 6.1-5.4(bs, 4H), 5.5-5.2(m, 2H), 4.7-3.5(m, 3H), 2.5-1.1 (m, 22H), 0.86(t, 3H) TanakaTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|