LBF20118BC02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
|Melting Point=66.3 - 67.6°C [[Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608|{{RelationTable/GetFirstAuthor|Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608}}]] | |Melting Point=66.3 - 67.6°C [[Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608|{{RelationTable/GetFirstAuthor|Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608}}]] | ||
|Boiling Point=203 - 205°C/2.5mmHg(Me ESTER) [[Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836}}]] | |Boiling Point=203 - 205°C/2.5mmHg(Me ESTER) [[Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836}}]] | ||
|UV Spectra= | |UV Spectra=lambdamax 217nm (epsilonmax 13,490) [[Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608|{{RelationTable/GetFirstAuthor|Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608}}]] | ||
|IR Spectra=C=C: 12. | |IR Spectra=C=C: 12.60mu, 13.52mu, 15.50mu [[Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Kalm_MJ:,J. Org. Chem.,1954,19,1836}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=2-Metyl-2-icosenoic acid was prepared from 2-methyl-2-icosenoic acid methyl [[Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608|{{RelationTable/GetFirstAuthor|Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608}}]]. | |Chemical Synthesis=2-Metyl-2-icosenoic acid was prepared from 2-methyl-2-icosenoic acid methyl [[Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608|{{RelationTable/GetFirstAuthor|Reference:Allen_CF:Kalm_MJ:,Org. Synth.,1963,4,608}}]]. | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA7104 |
| LipidMaps | LMFA01020136 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20118BC02 |
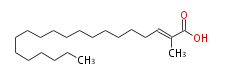
| 2-Methyl-2-Icosenoic Acid | |
|---|---|
| |
| Structural Information | |
| 2-Methyl-2-Icosenoic Acid | |
| |
| Formula | C21H40O2 |
| Exact Mass | 324.302830524 |
| Average Mass | 324.5411 |
| SMILES | C(CCCCCCCCCCCCCC=C(C)C(O)=O)CCC |
| Physicochemical Information | |
| 66.3 - 67.6°C Allen_CF et al. | |
| 203 - 205°C/2.5mmHg(Me ESTER) Cason_J et al. | |
| 2-Metyl-2-icosenoic acid was prepared from 2-methyl-2-icosenoic acid methyl Allen_CF et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | lambdamax 217nm (epsilonmax 13,490) Allen_CF et al. |
| IR Spectra | C=C: 12.60μ, 13.52μ, 15.50μ CasonJet al. |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|