LBF20207PG02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|SysName=7- [ 2- (3 (S) -Hydroxy-1 (E) -octenyl) -5-oxo-1-cyclopenten-1-yl ] heptanoic acid / (E,S) -15-Hydroxy-9-oxo-8 (12) ,13-prostadienoic acid | |SysName=7- [ 2- (3 (S) -Hydroxy-1 (E) -octenyl) -5-oxo-1-cyclopenten-1-yl ] heptanoic acid / (E,S) -15-Hydroxy-9-oxo-8 (12) ,13-prostadienoic acid | ||
|Common Name=&&PROSTAGLANDIN B1&& | |Common Name=&&PROSTAGLANDIN B1&& | ||
|Melting Point=70-71°C | |Melting Point=70-71°C [[Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231|{{RelationTable/GetFirstAuthor|Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231}}]] | ||
|Mass Spectra=METHYL ESTER ; m/e 350(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 332, 319, 301, 251, 219 | |Mass Spectra=METHYL ESTER ; m/e 350(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 332, 319, 301, 251, 219 [[Reference:Struijk_MCB:Beerthuis_RK:Pabon_HJJ:Van_Dorp_DA:,Recueil Travaux Quimiq Pays Bas,1966,85,1233|{{RelationTable/GetFirstAuthor|Reference:Struijk_MCB:Beerthuis_RK:Pabon_HJJ:Van_Dorp_DA:,Recueil Travaux Quimiq Pays Bas,1966,85,1233}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT> <SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 278nm(<FONT FACE="Symbol">e</FONT> 28,650) | |UV Spectra=<FONT FACE="Symbol">l</FONT> <SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 278nm(<FONT FACE="Symbol">e</FONT> 28,650) [[Reference:Jones_RL:,J. Lipid Res.,1972,13,511|{{RelationTable/GetFirstAuthor|Reference:Jones_RL:,J. Lipid Res.,1972,13,511}}]] | ||
|IR Spectra=5.91, 6.10, 6.27, 10.3<FONT FACE="Symbol">m</FONT>m | |IR Spectra=5.91, 6.10, 6.27, 10.3<FONT FACE="Symbol">m</FONT>m [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR :<FONT FACE="Symbol">d</FONT> 6.87(d, J=16Hz, 1H, 13-CH), 6.25(d,d, J=16.6Hz, 1H, 14-CH), 4.38(1H, 15-CH) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR :<FONT FACE="Symbol">d</FONT> 6.87(d, J=16Hz, 1H, 13-CH), 6.25(d,d, J=16.6Hz, 1H, 14-CH), 4.38(1H, 15-CH) [[Reference:Collins_P:Jung_CJ:Pappo_R:,Isr. J. Chem.,1968,6,839|{{RelationTable/GetFirstAuthor|Reference:Collins_P:Jung_CJ:Pappo_R:,Isr. J. Chem.,1968,6,839}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1100 |
| LipidMaps | LMFA03010131 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG02 |
| PROSTAGLANDIN B1 | |
|---|---|
| |
| Structural Information | |
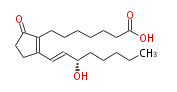
| 7- [ 2- (3 (S) -Hydroxy-1 (E) -octenyl) -5-oxo-1-cyclopenten-1-yl ] heptanoic acid / (E,S) -15-Hydroxy-9-oxo-8 (12) ,13-prostadienoic acid | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC[C@@H](O)C=CC(=C1CCCCCCC(O)=O)CCC(=O)1)CC |
| Physicochemical Information | |
| 70-71°C Ramwell_PW et al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 350(M+), 332, 319, 301, 251, 219 Struijk_MCB et al. |
| UV Spectra | l max = 278nm(e 28,650) Jones_RL |
| IR Spectra | 5.91, 6.10, 6.27, 10.3mm HambergMet al. |
| NMR Spectra | 1H-NMR :d 6.87(d, J=16Hz, 1H, 13-CH), 6.25(d,d, J=16.6Hz, 1H, 14-CH), 4.38(1H, 15-CH) CollinsPet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|