LBF20207PG02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR1100 | |LipidBank=XPR1100 | ||
|LipidMaps=LMFA03010131 | |LipidMaps=LMFA03010131 | ||
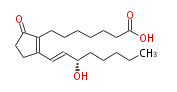
|SysName=7- [2- (3S -Hydroxy-1 | |SysName=7- [2- (3S -Hydroxy-trans-1-octenyl) -5-oxo-3-cyclopenten-1 -yl] heptanoic acid | ||
|Common Name=&&Prostaglandin B_1&&(E,S) -15-Hydroxy-9-oxo-8 (12) ,13-prostadienoic acid&&7- [ 2- (3S -Hydroxy-1(E)-octenyl) -5-oxo-3-cyclopenten-1 -yl ] heptanoic acid&& | |Common Name=&&Prostaglandin B_1&&(E,S) -15-Hydroxy-9-oxo-8 (12) ,13-prostadienoic acid&&7- [ 2- (3S -Hydroxy-1(E)-octenyl) -5-oxo-3-cyclopenten-1 -yl ] heptanoic acid&& | ||
|Melting Point=70-71°C [[Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231|{{RelationTable/GetFirstAuthor|Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231}}]] | |Melting Point=70-71°C [[Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231|{{RelationTable/GetFirstAuthor|Reference:Ramwell_PW:Shaw_JE:Clarke_GB:Grostic_MF:Kaiser_DG:Pike_JE:,Progr. Chem. Fats Other Lipids,1971,9,231}}]] | ||
Latest revision as of 08:27, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1100 |
| LipidMaps | LMFA03010131 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG02 |
| Prostaglandin B1 | |
|---|---|
| |
| Structural Information | |
| 7- [2- (3S -Hydroxy-trans-1-octenyl) -5-oxo-3-cyclopenten-1 -yl] heptanoic acid | |
| |
| PGB1 | |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC[C@@H](O)C=CC(=C1CCCCCCC(O)=O)CCC(=O)1)CC |
| Physicochemical Information | |
| 70-71°C Ramwell_PW et al. | |
| Prostaglandin B1 is found in human seminal plasma Bergstrom_S . | |
|
Collins_P et al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 350(M+), 332, 319, 301, 251, 219 Struijk_MCB et al. |
| UV Spectra | λ max = 278nm( ε 28,650) Jones_RL |
| IR Spectra | 5.91, 6.10, 6.27, 10.3 μ m HambergMet al. |
| NMR Spectra | 1H-NMR : δ 6.87(d, J=16Hz, 1H, 13-CH), 6.25(d,d, J=16.6Hz, 1H, 14-CH), 4.38(1H, 15-CH) CollinsPet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|