LBF20207PG25: Difference between revisions
m (LBF20307PG09 moved to LBF20207PG25) |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA03010002 | |LipidMaps=LMFA03010002 | ||
|SysName=7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |SysName=7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | ||
|Common Name=&&PROSTAGLANDIN | |Common Name=&&PROSTAGLANDIN F_2alpha&& | ||
|Melting Point=25-35°C [[Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123}}]] | |Melting Point=25-35°C [[Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123}}]] | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=23.8 °(C=1,THF) [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | |Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=23.8 °(C=1,THF) [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | ||
Revision as of 07:26, 19 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1501 |
| LipidMaps | LMFA03010002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG25 |
| PROSTAGLANDIN F_2α | |
|---|---|
| |
| Structural Information | |
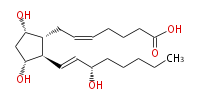
| 7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| Formula | C20H34O5 |
| Exact Mass | 354.240624198 |
| Average Mass | 354.48096000000004 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](C[C@@H]1O)O)CC |
| Physicochemical Information | |
| 25-35°C Bundy_GL et al. | |
| ETHYL ACETATE, ACETONE, DIETHYLETHER Pike_JEet al.. STABILITIES: to be stable under neutral and basic conditions Karim_SM et al. | |
| Spectral Information | |
| Mass Spectra | 354(M+), 336, 318, 292, 274, 264(100), 247, 229, 191, 177, 165, 137, 99, 81, 67 HorvathG |
| UV Spectra | |
| IR Spectra | NEAT : 3320, 2640, 1710, 1295, 1260, 1245, 1120, 1080, 1055, 1025, 975cm-1 Pike_JEet al. |
| NMR Spectra | 1H-NMR(d6-ACETONE) : d 5.48(m, 4H), 4.05(m, 3H), 0.9(t, 3H, 20-CH3) Pike_JEet al.. 13C-NMR : 176.6(C1), 135.0(C14), 132.8(C5), 129.1(C13 or C6), 128.9(C6 or C13), 77.2(C11), 72.9(C15), 71.8(C9), 55.0(C12), 49.9(C8), 42,6(C10), 36.8(C16), 33.2(C2), 31,5(C18), 26.3(C4), 25.1(C7), 25.1(C17), 24.5 LukacsGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|