LBF20207PG26: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1511 | |LipidBank=XPR1511 | ||
|LipidMaps=LMFA03010026 | |LipidMaps=LMFA03010026 | ||
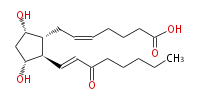
|SysName=7- [ | |SysName=7- [ (3R,5S) -Dihydroxy-2R - (3-oxo-trans-1-octenyl)- cyclopentan-1R -yl] -cis-5-heptenoic acid | ||
|Common Name=&&15- | |Common Name=&&15-keto Prostaglandin F_2 alpha&&7- [ (3R,5S) -Dihydroxy-2R - (3-oxo-1(E)-octenyl)- cyclopentan-1R -yl ] -5-(Z)-heptenoic acid&& | ||
|Source=15-Keto-prostaglandin F2 alpha is the oxidized product of prostaglandin F2 alpha by 15-hydroxyprostaglandin dehydrogenase, which is present in lung, kidney, placenta and other tissues and catalyzes the NAD- or NADP-dependent dehydrogenation of 15-dydroxyl group [[Reference:Hansen_HS:,Prostaglandins,1976,12,647|{{RelationTable/GetFirstAuthor|Reference:Hansen_HS:,Prostaglandins,1976,12,647}}]]. | |||
|Chemical Synthesis= | |||
|Metabolism=15-Keto-prostaglandin F2 alpha is further metabolized by its Delta 13-reduction, beta -oxidation and omega oxidation. The ultimate metabolite is 5 alpha ,7 alpha -dihydroxy-11-keto-tetranorprosta-1,16-dioic acid, and excreted in urine [[Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254|{{RelationTable/GetFirstAuthor|Reference:Granstrom_E:Samuelsson_B:,J. Biol. Chem.,1971,246,5254}}]]. | |||
|Symbol=15-KETO-PGF2α | |||
|Biological Activity=It is well known that the biological activities of various prostaglandins are reduced upon their dehydrogenation at carbon-15 by the catalysis of 15-hydroxyprostaglandin dehydrogenase [[Reference:Anggard_E:,Acta Physiol. Scand.,1966,66,509|{{RelationTable/GetFirstAuthor|Reference:Anggard_E:,Acta Physiol. Scand.,1966,66,509}}]]. | |||
|Genetic Information=cDNA for placental 15-hydroxyprostaglandin dehydrogenase was cloned [[Reference:Ensor_CM:Tai_HH:,J. Lipid Mediat. Cell Signal.,1995,12,313|{{RelationTable/GetFirstAuthor|Reference:Ensor_CM:Tai_HH:,J. Lipid Mediat. Cell Signal.,1995,12,313}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:30, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1511 |
| LipidMaps | LMFA03010026 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG26 |
| 15-keto Prostaglandin F2α | |
|---|---|
| |
| Structural Information | |
| 7- [ (3R,5S) -Dihydroxy-2R - (3-oxo-trans-1-octenyl)- cyclopentan-1R -yl] -cis-5-heptenoic acid | |
| |
| 15-KETO-PGF2α | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CCC(=O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](C[C@@H]1O)O)CC |
| Physicochemical Information | |
| 15-Keto-prostaglandin F2 alpha is the oxidized product of prostaglandin F2 alpha by 15-hydroxyprostaglandin dehydrogenase, which is present in lung, kidney, placenta and other tissues and catalyzes the NAD- or NADP-dependent dehydrogenation of 15-dydroxyl group Hansen_HS . | |
| 15-Keto-prostaglandin F2 alpha is further metabolized by its Delta 13-reduction, beta -oxidation and omega oxidation. The ultimate metabolite is 5 alpha ,7 alpha -dihydroxy-11-keto-tetranorprosta-1,16-dioic acid, and excreted in urine Granstrom_E et al.. | |
| It is well known that the biological activities of various prostaglandins are reduced upon their dehydrogenation at carbon-15 by the catalysis of 15-hydroxyprostaglandin dehydrogenase Anggard_E . | |
| cDNA for placental 15-hydroxyprostaglandin dehydrogenase was cloned Ensor_CM et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|