LBF20207PG50: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR8048 | |LipidBank=XPR8048 | ||
|LipidMaps=LMFA03010094 | |LipidMaps=LMFA03010094 | ||
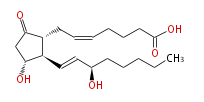
|SysName= ( | |SysName=(cis-5,trans-13) - (8R,11R,12R,15R) -11,15-Dihydroxy-9-oxoprost-5,13-dienoic acid | ||
|Common Name=&&15-epi | |Common Name=&&15-epi Prostaglandin E_2 acetate, methyl ester&&(5Z, 13E) - (8R,11R,12R,15R) -11,15-Dihydroxy-9-oxoprost-5,13-dienoic acid&& | ||
|Source=15-Epi-prostaglandin E_2 and its methyl ester were isolated from Gorgonian, Plexaura homomalla.[[Reference:Light_RJ:Samuelsson_B:,Eur. J. Biochem.,1972,28,232|{{RelationTable/GetFirstAuthor|Reference:Light_RJ:Samuelsson_B:,Eur. J. Biochem.,1972,28,232}}]] | |Source=15-Epi-prostaglandin E_2 and its methyl ester were isolated from Gorgonian, Plexaura homomalla.[[Reference:Light_RJ:Samuelsson_B:,Eur. J. Biochem.,1972,28,232|{{RelationTable/GetFirstAuthor|Reference:Light_RJ:Samuelsson_B:,Eur. J. Biochem.,1972,28,232}}]] | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Latest revision as of 08:37, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8048 |
| LipidMaps | LMFA03010094 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG50 |
| 15-epi Prostaglandin E2 acetate, methyl ester | |
|---|---|
| |
| Structural Information | |
| (cis-5,trans-13) - (8R,11R,12R,15R) -11,15-Dihydroxy-9-oxoprost-5,13-dienoic acid | |
| |
| (15R)-PGE_2 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 15-Epi-prostaglandin E_2 and its methyl ester were isolated from Gorgonian, Plexaura homomalla. Light_RJ et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|