LBF20207PG51: Difference between revisions
m (LBF20407PG01 moved to LBF20207PG51) |
No edit summary |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1801 | |LipidBank=XPR1801 | ||
|LipidMaps=LMFA03010087 | |LipidMaps=LMFA03010087 | ||
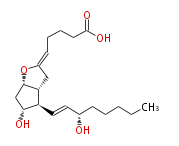
|SysName=5 | |SysName=cis-5- [ 7R -Hydroxy-6R - (3S -hydroxyocten-trans-1-yl) -(1S,5R)-2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | ||
|Common Name=&& | |Common Name=&&Prostaglandin I_2&&5 (Z) - [ 7 (R) -Hydroxy-6 (R) - (3 (S) -hydroxyocten-1 (E) -yl) -1 (S) ,5 (R) -2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid&& | ||
| | |Optical=[ alpha ]_D =78°(C=0.8820, CHCl3) [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|Mass Spectra=11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M | |Mass Spectra=11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M^+ -CH_3 ), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|IR Spectra=METHYL ESTER ; LIQUID MELT | |IR Spectra=METHYL ESTER ; LIQUID MELT nu 3370, 1740, 1695cm^{-1} [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 -H-NMR(D_2 O, GLYCINE BUFFER, pH10.4) : delta 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH_2 ) [[Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974}}]] | ||
|Source=In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin I2. Prostaglandin I2 is produced in blood vessels, lung and other tissues [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]]. | |||
|Chemical Synthesis=[[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] {{Image200|LBF20207PG51FT0001.gif}} | |||
|Metabolism=Prostaglandin I2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2 by the catalysis of prostaglandin I synthase [[Reference:Pace-Asciak_CR:Smith_WL:,The_Enzymes,1983,16,543|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Smith_WL:,The_Enzymes,1983,16,543}}]] [[Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243|{{RelationTable/GetFirstAuthor|Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243}}]]. Prostaglandin I2 is unstable and decomposes readily to 6-keto-prostaglandin F2 alpha . Its 2,3-dinor derivative is a major urinary metabolite [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]]. | |||
|Symbol=PGI2 | |||
|Biological Activity=Prostaglandin I2 is a potent anti-aggregatory agent for platelets, relaxes blood vessels, and enhances vascular permeability [[Reference:Whittle_BJ:Moncada_S:,Br. Med. Bull.,1983,39,232|{{RelationTable/GetFirstAuthor|Reference:Whittle_BJ:Moncada_S:,Br. Med. Bull.,1983,39,232}}]]. It binds to a receptor with 7 transmembrne domains (IP) coupled to a Gs protein [[Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109}}]]. | |||
|Genetic Information=cDNA [[Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243|{{RelationTable/GetFirstAuthor|Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243}}]] and genomic DNA [[Reference:Yokoyama_C:Yabuki_T:Inoue_H:Tone_Y:Hara_S:Hatae_T:Nagata_M:Takahashi_EI:Tanabe_T:,Genomics,1996,36,296|{{RelationTable/GetFirstAuthor|Reference:Yokoyama_C:Yabuki_T:Inoue_H:Tone_Y:Hara_S:Hatae_T:Nagata_M:Takahashi_EI:Tanabe_T:,Genomics,1996,36,296}}]] for prostaglandin I synthase were cloned. cDNA for IP was isolated [[Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Sugimoto_Y:Ichikawa_A:,Biochim. Biophys. Acta,1995,1259,109}}]]. | |||
|Note=Stability:unstable in water around neutrality with a half life of about 5 min at 37°C and decomposes to 6-keto-PGF1 alpha [[Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443|{{RelationTable/GetFirstAuthor|Reference:Negishi_M:Koizumi_T:Ichikawa_A:,J. Lipid Mediat. Cell Signal.,1995,12,443}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:40, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1801 |
| LipidMaps | LMFA03010087 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG51 |
| Prostaglandin I2 | |
|---|---|
| |
| Structural Information | |
| cis-5- [ 7R -Hydroxy-6R - (3S -hydroxyocten-trans-1-yl) -(1S,5R)-2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | |
| |
| PGI2 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]12)[C@H](O)C[C@H](OC(C2)=CCCCC(O)=O)1)CC |
| Physicochemical Information | |
| [ α ]D=78°(C=0.8820, CHCl3) Johnson_RA et al. | |
| In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin I2. Prostaglandin I2 is produced in blood vessels, lung and other tissues Moncada_S et al.. | |
Johnson_RA et al. 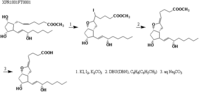 | |
| Prostaglandin I2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2 by the catalysis of prostaglandin I synthase Pace-Asciak_CR et al. Tanabe_T et al.. Prostaglandin I2 is unstable and decomposes readily to 6-keto-prostaglandin F2 alpha . Its 2,3-dinor derivative is a major urinary metabolite Needleman_P et al.. | |
| Prostaglandin I2 is a potent anti-aggregatory agent for platelets, relaxes blood vessels, and enhances vascular permeability Whittle_BJ et al.. It binds to a receptor with 7 transmembrne domains (IP) coupled to a Gs protein Negishi_M et al.. | |
| cDNA Tanabe_T et al. and genomic DNA Yokoyama_C et al. for prostaglandin I synthase were cloned. cDNA for IP was isolated Negishi_M et al.. | |
| Stability:unstable in water around neutrality with a half life of about 5 min at 37°C and decomposes to 6-keto-PGF1 alpha Negishi_M et al.. | |
| Spectral Information | |
| Mass Spectra | 11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M+-CH3), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 Johnson_RA et al. |
| UV Spectra | |
| IR Spectra | METHYL ESTER ; LIQUID MELT ν 3370, 1740, 1695cm-1 Johnson_RA et al. |
| NMR Spectra | 1-H-NMR(D2O, GLYCINE BUFFER, pH10.4) : δ 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH2) KotovychGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|