LBF20207PG79
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1767 |
| LipidMaps | LMFA03010085 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG79 |
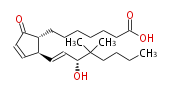
| 16,16-dimethyl Prostaglandin A1 | |
|---|---|
| |
| Structural Information | |
| 9-Oxo-15R-hydroxy-16,16-dimethyl-prost- (10,trans-13) -dien-1-oic acid | |
| |
| Formula | C22H36O4 |
| Exact Mass | 364.26135963999997 |
| Average Mass | 364.51884 |
| SMILES | C(C(C)(C)[C@H](C=C[C@@H](C=1)[C@@H](CCCCCCC(O)=O)C(C1)=O)O)CCC |
| Physicochemical Information | |
| 16,16-dimethyl Prostaglandin A1 is soluble in organic solvents (i.e. methyl acetate, DMSO, ethanol) at least 50 mg/ml and also in aqueous buffers or isotonic saline at least 2 mg/ml. But in basic solutions (pH >7.4) 16,16-dimethyl Prostaglandin A1 will be converted into 16,16-dimethyl Prostaglandin PGB1. | |
| 16,16-dimethyl Prostaglandin A1 is a metabolism resistant analogue of PGA1. | |
| 16,16-dimethyl Prostaglandin A1 is a metabolism resistant analogue of PGA1. | |
| 16,16-dimethyl Prostaglandin A1 shows inhibition against infection of HSV and HIV-1 at the ID50 of 3.8-7.3 and 2.5 mu g/ml respectively. Hughes-Fulford_M et al. 16,16-dimethyl Prostaglandin A1 shows dose-dependent inhibition in growth of human oral squamous carcinoma cells. ElAttar_TM et al. 16,16-dimethyl Prostaglandin A1 shows cell cycle arrest at the G1/S phase due to nhibiting DNA synthesis. Hughes-Fulford_M | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max=216nm ε 216=13000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|