LBF20207TX03: Difference between revisions
m (LBF20307TX03 moved to LBF20207TX03) |
m (LBF20307TX03 moved to LBF20207TX03) |
||
| Line 10: | Line 10: | ||
|Solubility=METHANOL, ETHYL ACETATE [[Reference:Roberts_LJ:2nd:Sweetman_BJ:Oates_JA:,J. Biol. Chem.,1978,253,5305|{{RelationTable/GetFirstAuthor|Reference:Roberts_LJ:2nd:Sweetman_BJ:Oates_JA:,J. Biol. Chem.,1978,253,5305}}]] | |Solubility=METHANOL, ETHYL ACETATE [[Reference:Roberts_LJ:2nd:Sweetman_BJ:Oates_JA:,J. Biol. Chem.,1978,253,5305|{{RelationTable/GetFirstAuthor|Reference:Roberts_LJ:2nd:Sweetman_BJ:Oates_JA:,J. Biol. Chem.,1978,253,5305}}]] | ||
|Mass Spectra=METHYL ESTER BIS-TMS ETHER ; m/e 526(M^+ ), 511, 455, 370, 295 [[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | |Mass Spectra=METHYL ESTER BIS-TMS ETHER ; m/e 526(M^+ ), 511, 455, 370, 295 [[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | ||
|IR Spectra=METHYL ESTER ; CHLOROFORM solution, nu 1730 cm^- ^1 [[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | |IR Spectra=METHYL ESTER ; CHLOROFORM solution, nu 1730 cm^- ^1 [[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | ||
|NMR Spectra=METHYL ESTER ; ^1 H-NMR(CDCl_3 ) : delta 5.86-5.78(m, 2H), 5.56-5.32(m, 2H), 5.13-4.72(m, 1H), 5.23-4.05(m, 2H), 3.67(S, 3H, OCH_3 )[[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | |NMR Spectra=METHYL ESTER ; ^1 H-NMR(CDCl_3 ) : delta 5.86-5.78(m, 2H), 5.56-5.32(m, 2H), 5.13-4.72(m, 1H), 5.23-4.05(m, 2H), 3.67(S, 3H, OCH_3 )[[Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85|{{RelationTable/GetFirstAuthor|Reference:Nelson_NA:Jackson_RW:Sebek_OK:,Prostaglandins,1978,16,85}}]] | ||
|Source=When thromboxane B2 is infused, 11-dehydro-thromboxane B2 is found as a major metabolite in the blood of rabbit [[Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413}}]] and man [[Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929}}]]. The compound appears in urine as one of the mejor metabolites [[Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929}}]][[Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413}}]]. | |Source=When thromboxane B2 is infused, 11-dehydro-thromboxane B2 is found as a major metabolite in the blood of rabbit [[Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413}}]] and man [[Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929}}]]. The compound appears in urine as one of the mejor metabolites [[Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Granstrom_E:Kumlin_M:Nordenstrom_A:,Prostaglandins,1986,31,929}}]][[Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413|{{RelationTable/GetFirstAuthor|Reference:Westlund_P:Kumlin_M:Nordenstrom_A:Granstrom_E:,Prostaglandins,1986,31,413}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR2121 |
| LipidMaps | LMFA03030004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207TX03 |
| 11-DEHYDROTHROMBOXANE B2 | |
|---|---|
| |
| Structural Information | |
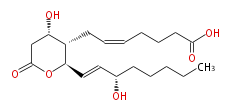
| 7- [ Tetrahydro-4 (S) -hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -6-oxo-2H-pyran-3 (S) -yl ] -5 (Z) -heptenoic acid | |
| |
| 11-DEHYDRO-TXB2 | |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CC[C@@H](O)C=C[C@@H](O1)[C@H]([C@H](CC1=O)O)CC=CCCCC(O)=O)CC |
| Physicochemical Information | |
| METHANOL, ETHYL ACETATE Roberts_LJ et al. | |
| When thromboxane B2 is infused, 11-dehydro-thromboxane B2 is found as a major metabolite in the blood of rabbit Westlund_P et al. and man Westlund_P et al.. The compound appears in urine as one of the mejor metabolites Westlund_P et al. Westlund_P et al.. | |
| 11-Hydroxythromboxane B2 dehydrogenase is considered to be the enzyme responsible for 11-dehydro-thromboxane B2 production Westlund_P et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER BIS-TMS ETHER ; m/e 526(M+), 511, 455, 370, 295 Nelson_NA et al. |
| UV Spectra | |
| IR Spectra | METHYL ESTER ; CHLOROFORM solution, ν 1730 cm-1 Nelson_NA et al. |
| NMR Spectra | METHYL ESTER ; 1H-NMR(CDCl3) : δ 5.86-5.78(m, 2H), 5.56-5.32(m, 2H), 5.13-4.72(m, 1H), 5.23-4.05(m, 2H), 3.67(S, 3H, OCH3) Nelson_NA et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|