LBF20303PG06: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR1725 | |LipidBank=XPR1725 | ||
|LipidMaps=LMFA03010045 | |LipidMaps=LMFA03010045 | ||
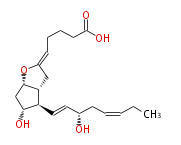
|SysName=6,9alpha-Epoxy-11alpha,15S-dihydroxy-prosta- | |SysName=(6,9alpha) -Epoxy- (11alpha,15S) -dihydroxy-prosta- (5-cis,13-trans,17-cis) -trien-1-oic acid | ||
|Common Name=&&Prostaglandin I_3&& | |Common Name=&&Prostaglandin I_3&&(6,9alpha) -Epoxy- (11alpha,15S) -dihydroxy-prosta- (5Z,13E,17Z) -trien-1-oic acid&& | ||
|Source=PGI3 is synthesized from EPA by cyclooxygenase and PGI synthase. | |Source=PGI3 is synthesized from EPA by cyclooxygenase and PGI synthase. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 07:35, 27 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1725 |
| LipidMaps | LMFA03010045 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20303PG06 |
| Prostaglandin I3 | |
|---|---|
| |
| Structural Information | |
| (6,9α) -Epoxy- (11α,15S) -dihydroxy-prosta- (5-cis,13-trans,17-cis) -trien-1-oic acid | |
| |
| Formula | C20H30O5 |
| Exact Mass | 350.20932407 |
| Average Mass | 350.4492 |
| SMILES | C(=CC[C@@H](O)C=C[C@H]([C@H]12)[C@H](O)C[C@H](O[C@@H](C2)C=CCCC(O)=O)1)CC |
| Physicochemical Information | |
| PGI3 is synthesized from EPA by cyclooxygenase and PGI synthase. | |
| PGI3 has equal ability to PGI2 in inhibitting human platelet aggregation. Mann_NJ et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|