LBF20303TX01: Difference between revisions
m (LBF20403TX01 moved to LBF20303TX01) |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR2002 | |LipidBank=XPR2002 | ||
|LipidMaps=LMFA03030005 | |LipidMaps=LMFA03030005 | ||
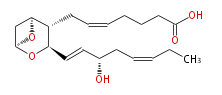
|SysName=7- [ 3- ( | |SysName=7- [3- (3S-Hydroxy- (trans-1,cis-5) -octadienyl) -(1S,5S,4,6) -dioxabicyclo [ 3.1.1 ] hept-2-yl] -cis-5-heptenoic acid | ||
|Common Name=&& | |Common Name=&&Thromboxane A_3&&7- [3- (3S -Hydroxy- (1E,5Z) -octadienyl) -(1S,5S,4,6) -dioxabicyclo [ 3.1.1 ] hept-2-yl] -5(Z) -heptenoic acid&& | ||
|Source= | |||
|Chemical Synthesis= | |||
|Metabolism=Prostaglandin H3 is produced from 5,8,11,14,17-eicosapentaenoic acid, which is one-eighth as efficient a substrate as arachidonic acid, by the catalysis of fatty acid cyclooxygenase, and then transformed to unstable thromboxane A3 [[Reference:Needleman_P:Raz_A:Minkes_MS:Ferrendelli_JA:Sprecher_H:,Proc. Natl. Acad. Sci. U. S. A.,1979,76,944|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Raz_A:Minkes_MS:Ferrendelli_JA:Sprecher_H:,Proc. Natl. Acad. Sci. U. S. A.,1979,76,944}}]], which is converted non-enzymatically to stable thromboxane B3. | |||
|Symbol=TXA3 | |||
|Biological Activity=Thromboxane A3 did not cause platelet aggregation unlike thromboxane A2, and inhibited platelet aggregation by other agonists [[Reference:Needleman_P:Raz_A:Minkes_MS:Ferrendelli_JA:Sprecher_H:,Proc. Natl. Acad. Sci. U. S. A.,1979,76,944|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Raz_A:Minkes_MS:Ferrendelli_JA:Sprecher_H:,Proc. Natl. Acad. Sci. U. S. A.,1979,76,944}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 07:06, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR2002 |
| LipidMaps | LMFA03030005 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20303TX01 |
| Thromboxane A3 | |
|---|---|
| |
| Structural Information | |
| 7- [3- (3S-Hydroxy- (trans-1,cis-5) -octadienyl) -(1S,5S,4,6) -dioxabicyclo [ 3.1.1 ] hept-2-yl] -cis-5-heptenoic acid | |
| |
| TXA3 | |
| Formula | C21H32O4 |
| Exact Mass | 348.23005951199997 |
| Average Mass | 348.47637999999995 |
| SMILES | C([C@H]([C@H](C=C[C@H](O)CC=CCC)2)[C@@H](C1)O[C@H](C2)1)C=CCCCC(O)=O |
| Physicochemical Information | |
| Prostaglandin H3 is produced from 5,8,11,14,17-eicosapentaenoic acid, which is one-eighth as efficient a substrate as arachidonic acid, by the catalysis of fatty acid cyclooxygenase, and then transformed to unstable thromboxane A3 Needleman_P et al., which is converted non-enzymatically to stable thromboxane B3. | |
| Thromboxane A3 did not cause platelet aggregation unlike thromboxane A2, and inhibited platelet aggregation by other agonists Needleman_P et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|