LBF20306EO02: Difference between revisions
No edit summary |
No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8111 | |LipidBank=DFA8111 | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName= dl-8 (9) - | |SysName=dl-8 (9) -Epoxy- (cis-5,cis-11,cis-14) -eicosatrienoic acid | ||
|Common Name=&&(+-) -8 (9) - | |Common Name=&&(+-) -8 (9) -Epoxy- (5Z,11Z,14Z) -eicosatrienoic acid&& | ||
|Source=(±)8(9)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771}}]][[Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916|{{RelationTable/GetFirstAuthor|Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916}}]]. (±)8(9)-EpETrE is a major P450 metabolite in the renal cortex [[Reference:Zhang_JY:Prakash_C:Yamashita_K:Blair_IA:,Biochem. Biophys. Res. Commun.,1992,183,138|{{RelationTable/GetFirstAuthor|Reference:Zhang_JY:Prakash_C:Yamashita_K:Blair_IA:,Biochem. Biophys. Res. Commun.,1992,183,138}}]]. | |Source=(±)8(9)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 [[Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771|{{RelationTable/GetFirstAuthor|Reference:Oliw_EH:Guengerich_FP:Oates_JA:,J. Biol. Chem.,1982,257,3771}}]][[Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916|{{RelationTable/GetFirstAuthor|Reference:Chacos_N:Falck_JR:Wixtrom_C:Capdevila_J:,Biochem. Biophys. Res. Commun.,1982,104,916}}]]. (±)8(9)-EpETrE is a major P450 metabolite in the renal cortex [[Reference:Zhang_JY:Prakash_C:Yamashita_K:Blair_IA:,Biochem. Biophys. Res. Commun.,1992,183,138|{{RelationTable/GetFirstAuthor|Reference:Zhang_JY:Prakash_C:Yamashita_K:Blair_IA:,Biochem. Biophys. Res. Commun.,1992,183,138}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Symbol=( | |Symbol=(+-)8(9)-EpETrE | ||
|Biological Activity=(±)8(9)-EpETrE reduces the glomerular filtration rate (GFR) through cyclooxygenase-dependent preglomerular vasoconstriction [[Reference:Katoh_T:Takahashi_K:Capdevila_J:Karara_A:Falck:JR:Jacobson:HR:Badr:KF:,Am. J. Physiol.,1991,261,F578|{{RelationTable/GetFirstAuthor|Reference:Katoh_T:Takahashi_K:Capdevila_J:Karara_A:Falck:JR:Jacobson:HR:Badr:KF:,Am. J. Physiol.,1991,261,F578}}]]>.(±)8(9)-EpETrE has been shown to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells [[Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546|{{RelationTable/GetFirstAuthor|Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546}}]]. | |Biological Activity=(±)8(9)-EpETrE reduces the glomerular filtration rate (GFR) through cyclooxygenase-dependent preglomerular vasoconstriction [[Reference:Katoh_T:Takahashi_K:Capdevila_J:Karara_A:Falck:JR:Jacobson:HR:Badr:KF:,Am. J. Physiol.,1991,261,F578|{{RelationTable/GetFirstAuthor|Reference:Katoh_T:Takahashi_K:Capdevila_J:Karara_A:Falck:JR:Jacobson:HR:Badr:KF:,Am. J. Physiol.,1991,261,F578}}]]>.(±)8(9)-EpETrE has been shown to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells [[Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546|{{RelationTable/GetFirstAuthor|Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546}}]]. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 06:15, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8111 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20306EO02 |
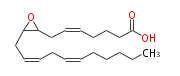
| (+-) -8 (9) -Epoxy- (5Z,11Z,14Z) -eicosatrienoic acid | |
|---|---|
| |
| Structural Information | |
| dl-8 (9) -Epoxy- (cis-5,cis-11,cis-14) -eicosatrienoic acid | |
| |
| (+-)8(9)-EpETrE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CCC=CCC(O1)C1CC=CCCCC(O)=O)CCC |
| Physicochemical Information | |
| (±)8(9)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 Oliw_EH et al. Chacos_N et al.. (±)8(9)-EpETrE is a major P450 metabolite in the renal cortex Zhang_JY et al.. | |
| (±)8(9)-EpETrE reduces the glomerular filtration rate (GFR) through cyclooxygenase-dependent preglomerular vasoconstriction Katoh_T et al.>.(±)8(9)-EpETrE has been shown to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells Graber_MN et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|