LBF20306EO03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
|Symbol=(+-)11(12)-EpETrE | |Symbol=(+-)11(12)-EpETrE | ||
|Biological Activity=(±)11(12)-EpETrE has been shown, along with (±)8(9)-EpETrE, to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells [[Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546|{{RelationTable/GetFirstAuthor|Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546}}]]. The compound relaxes intestinal artery, inhibits vasopressin-dependent water flow in urinary bladder, and regulates intracellular calcium level [[Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229|{{RelationTable/GetFirstAuthor|Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229}}]]<!--0078-->. | |Biological Activity=(±)11(12)-EpETrE has been shown, along with (±)8(9)-EpETrE, to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells [[Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546|{{RelationTable/GetFirstAuthor|Reference:Graber_MN:Alfonso_A:Gill_DL:,J. Biol. Chem.,1997,272,29546}}]]. The compound relaxes intestinal artery, inhibits vasopressin-dependent water flow in urinary bladder, and regulates intracellular calcium level [[Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229|{{RelationTable/GetFirstAuthor|Reference:Fitzpatrick_FA:Murphy_RC:,Pharmacol._Rev.,1988,40,229}}]]<!--0078-->. | ||
}} | |||
{{MassbankSpectra| | |||
UT000001 | |||
UT000002 | |||
UT000003 | |||
UT000004 | |||
UT000005 | |||
UT000006 | |||
UT000007 | |||
UT000008 | |||
UT000009 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:00, 21 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6302 DFA8112, XPR6302 |
| LipidMaps | LMFA03080004 |
| CAS | 81276-02-0 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20306EO03 |
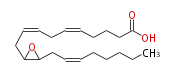
| (+-) -11 (12) -Epoxy- (5Z,8Z,14Z) -eicosatrienoic acid | |
|---|---|
| |
| Structural Information | |
| dl-11 (12) -Epoxy- (cis-5,cis-8,cis-14) -eicosatrienoic acid | |
| |
| (+-)11(12)-EpETrE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CCC(O1)C1CC=CCC=CCCCC(O)=O)CCC |
| Physicochemical Information | |
| 11(R),12(S)-EET METHYL ESTER ; [ α ]23 D =+4.94°(C=1.64, ACETONE) MossetPet al., 11(S),12(R)-EET METHYL ESTER ; [ α ]24 D =-2.34°(C=0.66, CHLOROFORM) Falck_JR et al. | |
| (±)11(12)-EpETrE is biosynthesized in rat and rabbit liver microsomes by cytochrome P450 Oliw_EH et al. Chacos_N et al.. The compound is produced when arachidonic acid in the presence of NADPH is incubated with the liver microsome of rat Chacos_N et al. or rabbit Oliw_EH et al. | |
| Table0001 Mosset_P et al. | |
| (±)11(12)-EpETrE has been shown, along with (±)8(9)-EpETrE, to play a role in the recovery of depleted Ca^{2+} pools in cultured smooth muscle cells Graber_MN et al.. The compound relaxes intestinal artery, inhibits vasopressin-dependent water flow in urinary bladder, and regulates intracellular calcium level Fitzpatrick_FA et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 340(M+), 322, 309, 227, 155 Oliw_EH et al.. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H-NMR(CDCl3) : δ 5.54-5.20(m, 6H), 3.61(s, 3H), 3.00-2.68(m, 4H), 2.37-2.00(m, 10H), 1.81-1.54(m, 2H), 1.46-1.13(m, 6H), 0.88(t, J=7Hz, 3H) MossetPet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








