LBF20306PG01: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=XPR1733 | |LipidBank=XPR1733 | ||
|LipidMaps=LMFA03010051 | |LipidMaps=LMFA03010051 | ||
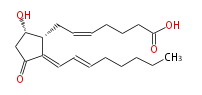
|SysName= 9alpha- | |SysName=9alpha-Hydroxy-11-oxo-prosta- (cis-5,trans-12,trans-14) -trien-1-oic acid | ||
|Common Name=&& 15-deoxy-Delta^{12.14}-Prostaglandin D_2&&9alpha- | |Common Name=&&15-deoxy-Delta^{12.14}- Prostaglandin D_2&&9alpha-Hydroxy-11-oxo-prosta- (5Z,12E,14E) -trien-1-oic acid&& | ||
|UV Spectra= | |UV Spectra= lambda max=296nm epsilon 296=18300 | ||
|Chromatograms=PGD2 and other metabolites are separated with HPLC. Please reffer following paper.[[Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35|{{RelationTable/GetFirstAuthor|Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35}}]] | |Chromatograms=PGD2 and other metabolites are separated with HPLC. Please reffer following paper.[[Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35|{{RelationTable/GetFirstAuthor|Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35}}]] | ||
|Source= | |||
|Chemical Synthesis= | |||
|Metabolism=15-deoxy- Delta ^{12.14}-Prostaglandin D2 is formed from Prostaglandin D2 via Delta ^{12}-Prostaglandin D2. This reaction is proceeded under co-culture with Prostaglandin D2 and serum.[[Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35|{{RelationTable/GetFirstAuthor|Reference:Soderstrom_M:Wigren_J:Surapureddi_S:Glass_CK:Hammarstrom_S:,Biochim. Biophys. Acta,2003,1631,35}}]] | |||
|Biological Activity= 15-deoxy- Delta ^{12.14}-Prostaglandin D2 shows cytotoxity on murine leukemia cell line (IC50 value of 0.3 mu g/ml).[[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] 15-deoxy- Delta ^{12.14}-Prostaglandin D2 is a potent activator of eosinophils, inducing calcium mobilization, actin polymerization, and CD11b expression.[[Reference:Monneret_G:Li_H:Vasilescu_J:Rokach_J:Powell_WS:,J. Immunol.,2002,168,3563|{{RelationTable/GetFirstAuthor|Reference:Monneret_G:Li_H:Vasilescu_J:Rokach_J:Powell_WS:,J. Immunol.,2002,168,3563}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 09:00, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1733 |
| LipidMaps | LMFA03010051 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20306PG01 |
| 15-deoxy-Δ12.14- Prostaglandin D2 | |
|---|---|
| |
| Structural Information | |
| 9α-Hydroxy-11-oxo-prosta- (cis-5,trans-12,trans-14) -trien-1-oic acid | |
| |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C([C@H]1CC=CCCCC(O)=O)(C(C[C@@H]1O)=O)=CC=CCCCCC |
| Physicochemical Information | |
| 15-deoxy- Delta ^{12.14}-Prostaglandin D2 is formed from Prostaglandin D2 via Delta ^{12}-Prostaglandin D2. This reaction is proceeded under co-culture with Prostaglandin D2 and serum. Soderstrom_M et al. | |
| 15-deoxy- Delta ^{12.14}-Prostaglandin D2 shows cytotoxity on murine leukemia cell line (IC50 value of 0.3 mu g/ml). Corey_EJ et al. 15-deoxy- Delta ^{12.14}-Prostaglandin D2 is a potent activator of eosinophils, inducing calcium mobilization, actin polymerization, and CD11b expression. Monneret_G et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max=296nm ε 296=18300 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | PGD2 and other metabolites are separated with HPLC. Please reffer following paper. SoderstromMet al. |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|