LBF20307PG04: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR1131 | |LipidBank=XPR1131 | ||
|LipidMaps=LMFA03010132 | |LipidMaps=LMFA03010132 | ||
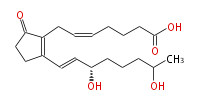
|SysName=7- [ | |SysName=7- [2R- ((3S,7)-Dihydroxy-trans-1-octenyl) -5-oxo-1-cyclopenten-1R-yl] -cis-5-heptenoic acid | ||
|Common Name=&&19-hydroxy Prostaglandin B_2&&(15S,19)-Dihydroxy-9-oxo-5-cis-8(12),13-trans-prostadienoic acid&&7- [2R- ((3S,7)-Dihydroxy-1-(E)-octenyl) -5-oxo-1-cyclopenten-1R-yl] -5-(Z)-heptenoic acid | |||
|Solubility=ETHANOL [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | |Solubility=ETHANOL [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | ||
|UV Spectra= | |UV Spectra= lambda ^{EtOH}_{max} = 278 nm( epsilon ∼20,000) [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | ||
|IR Spectra= 5.92, 6.09, 6.26, 10.3 | |IR Spectra= 5.92, 6.09, 6.26, 10.3 mu m [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | ||
|Source=The compound is contained in human seminal plasma in a considerable amount [[Reference:Bergstrom_S:,Science,1967,157,382|{{RelationTable/GetFirstAuthor|Reference:Bergstrom_S:,Science,1967,157,382}}]], but it is attributed presumably to a degradation product of 19-hydroxy-prostagaldnin E2 [[Reference:Taylor_PL:Kelly_RW:,Nature,1974,250,665|{{RelationTable/GetFirstAuthor|Reference:Taylor_PL:Kelly_RW:,Nature,1974,250,665}}]]. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Symbol=19-HYDROXY-PGB2 | |||
|Biological Activity=19-Hydroxy-prostaglandin B2 relaxes uterine myometriuim [[Reference:Horton_EW:,Physiol. Rev.,1969,49,122|{{RelationTable/GetFirstAuthor|Reference:Horton_EW:,Physiol. Rev.,1969,49,122}}]]. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 09:01, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1131 |
| LipidMaps | LMFA03010132 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG04 |
| 19-hydroxy Prostaglandin B2 | |
|---|---|
| |
| Structural Information | |
| 7- [2R- ((3S,7)-Dihydroxy-trans-1-octenyl) -5-oxo-1-cyclopenten-1R-yl] -cis-5-heptenoic acid | |
| |
| 19-HYDROXY-PGB2 | |
| Formula | C20H30O5 |
| Exact Mass | 350.20932407 |
| Average Mass | 350.4492 |
| SMILES | O[C@H](CCC[C@H](C)O)C=CC(=C1CC=CCCCC(O)=O)CCC(=O)1 |
| Physicochemical Information | |
| ETHANOL HambergMet al. | |
| The compound is contained in human seminal plasma in a considerable amount Bergstrom_S , but it is attributed presumably to a degradation product of 19-hydroxy-prostagaldnin E2 Taylor_PL et al.. | |
| 19-Hydroxy-prostaglandin B2 relaxes uterine myometriuim Horton_EW . | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ EtOH max = 278 nm( ε ∼20,000) HambergMet al. |
| IR Spectra | 5.92, 6.09, 6.26, 10.3 μ m HambergMet al. |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|