LBF20307PG05: Difference between revisions
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
|NMR Spectra=METHYL ESTER ; <FONT FACE="Symbol">d</FONT> 6.3(d, J=16Hz, 1H, 13-CH), 6.1-5.95(m, 2H, 11-CH), 5,7(dd, J=6,16Hz, 1H, 14-CH), 5.7-5.1(m, 2H, 5,6-CH), 4.4-4.0(m, 1H, 15-CH), 3.69(S, 3H, OCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 3.3-3.0(m, 1H,8-CH), 3.0-2.8(m, 2H, 10-CH) [[Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903|{{RelationTable/GetFirstAuthor|Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903}}]] | |NMR Spectra=METHYL ESTER ; <FONT FACE="Symbol">d</FONT> 6.3(d, J=16Hz, 1H, 13-CH), 6.1-5.95(m, 2H, 11-CH), 5,7(dd, J=6,16Hz, 1H, 14-CH), 5.7-5.1(m, 2H, 5,6-CH), 4.4-4.0(m, 1H, 15-CH), 3.69(S, 3H, OCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 3.3-3.0(m, 1H,8-CH), 3.0-2.8(m, 2H, 10-CH) [[Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903|{{RelationTable/GetFirstAuthor|Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=[[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]];> {{Image200| | |Chemical Synthesis=[[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]];> {{Image200|LBF20307PG05FT0001.gif}} | ||
|Metabolism=Prostaglandin A isomerase converts prostaglandin A2 to C2, and the enzyme is found in human serum [[Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351|{{RelationTable/GetFirstAuthor|Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351}}]];> and in the plasma of rabbit, cat, pig, dog and rat [[Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588|{{RelationTable/GetFirstAuthor|Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588}}]];>. | |Metabolism=Prostaglandin A isomerase converts prostaglandin A2 to C2, and the enzyme is found in human serum [[Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351|{{RelationTable/GetFirstAuthor|Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351}}]];> and in the plasma of rabbit, cat, pig, dog and rat [[Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588|{{RelationTable/GetFirstAuthor|Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588}}]];>. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 10:00, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1201 |
| LipidMaps | LMFA03010133 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG05 |
| PROSTAGLANDIN C2 | |
|---|---|
| |
| Structural Information | |
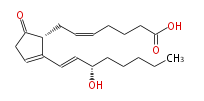
| 7- [ 2- (3 (S) -Hydroxy-1 (E) -octenyl) -5-oxo-2-cyclopenten-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)C=CC([C@H]1CC=CCCCC(O)=O)=CCC(=O)1)CC |
| Physicochemical Information | |
| METHANOL, CHLOROFORM Corey_EJ et al. | |
Corey_EJ et al.;> 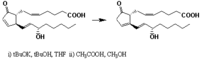 | |
| Prostaglandin A isomerase converts prostaglandin A2 to C2, and the enzyme is found in human serum Polet_H et al.;> and in the plasma of rabbit, cat, pig, dog and rat Jones_RL et al.;>. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER; m/e 348(M+), 330, 249, 245, 217, 215, 190, 133, 119, 109 Floyd_MB et al. |
| UV Spectra | l MeOHmax = 234 nm (e 17000) Corey_EJ et al. |
| IR Spectra | CHLOROFORM solution, n 1750,1715 cm-1 Corey_EJ et al. |
| NMR Spectra | METHYL ESTER ; d 6.3(d, J=16Hz, 1H, 13-CH), 6.1-5.95(m, 2H, 11-CH), 5,7(dd, J=6,16Hz, 1H, 14-CH), 5.7-5.1(m, 2H, 5,6-CH), 4.4-4.0(m, 1H, 15-CH), 3.69(S, 3H, OCH3), 3.3-3.0(m, 1H,8-CH), 3.0-2.8(m, 2H, 10-CH) Floyd_MB et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|