LBF20307PG05: Difference between revisions
No edit summary |
No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=XPR1201 | |LipidBank=XPR1201 | ||
|LipidMaps=LMFA03010133 | |LipidMaps=LMFA03010133 | ||
|SysName=7- [ | |SysName=7- [2R- (3S-hydroxy-trans-1-octenyl) -5-oxo-2-cyclopenten-1R-yl] -cis-5-heptenoic acid | ||
|Common Name=&& | |Common Name=&&Prostaglandin C_2&&7- [2R- (3S-Hydroxy-1-(E)-octenyl) -5-oxo-2-cyclopenten-1R-yl] -5-(Z)-heptenoic acid&& | ||
|Solubility=METHANOL, CHLOROFORM [[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] | |Solubility=METHANOL, CHLOROFORM [[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] | ||
|Mass Spectra=METHYL ESTER; m/e 348(M | |Mass Spectra=METHYL ESTER; m/e 348(M^+ ), 330, 249, 245, 217, 215, 190, 133, 119, 109 [[Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903|{{RelationTable/GetFirstAuthor|Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903}}]] | ||
|UV Spectra= | |UV Spectra= lambda ^{MeOH}_{max} = 234 nm (epsilon ∼ 17000) [[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] | ||
|IR Spectra=CHLOROFORM solution, | |IR Spectra=CHLOROFORM solution, nu 1750,1715 cm^{-1} [[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] | ||
|NMR Spectra=METHYL ESTER ; | |NMR Spectra=METHYL ESTER ; delta 6.3(d, J=16Hz, 1H, 13-CH), 6.1-5.95(m, 2H, 11-CH), 5,7(dd, J=6,16Hz, 1H, 14-CH), 5.7-5.1(m, 2H, 5,6-CH), 4.4-4.0(m, 1H, 15-CH), 3.69(S, 3H, OCH_3 ), 3.3-3.0(m, 1H,8-CH), 3.0-2.8(m, 2H, 10-CH) [[Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903|{{RelationTable/GetFirstAuthor|Reference:Floyd_MB:Schaub_RE:Siuta_GJ:Skotnicki_JS:Grudzinskas_CV:Weiss_MJ:Dessy_F:VanHumbeeck_L:,J. Med. Chem.,1980,23,903}}]] | ||
|Source= | |||
|Chemical Synthesis=[[Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Cyr_CR:,Tetrah. Lett.,1974,,1761}}]] {{Image200|LBF20307PG05FT0001.gif}} | |||
|Metabolism=Prostaglandin A isomerase converts prostaglandin A2 to C2, and the enzyme is found in human serum [[Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351|{{RelationTable/GetFirstAuthor|Reference:Polet_H:Levine_L:,J. Biol. Chem.,1975,250,351}}]] and in the plasma of rabbit, cat, pig, dog and rat [[Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588|{{RelationTable/GetFirstAuthor|Reference:Jones_RL:Cammock_S:Horton_EW:,Biochim. Biophys. Acta,1972,280,588}}]]. | |||
|Symbol=PGC2 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 08:19, 4 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1201 |
| LipidMaps | LMFA03010133 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG05 |
| Prostaglandin C2 | |
|---|---|
| |
| Structural Information | |
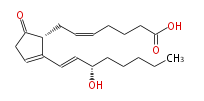
| 7- [2R- (3S-hydroxy-trans-1-octenyl) -5-oxo-2-cyclopenten-1R-yl] -cis-5-heptenoic acid | |
| |
| PGC2 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)C=CC([C@H]1CC=CCCCC(O)=O)=CCC(=O)1)CC |
| Physicochemical Information | |
| METHANOL, CHLOROFORM Corey_EJ et al. | |
Corey_EJ et al. 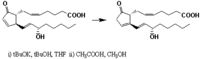 | |
| Prostaglandin A isomerase converts prostaglandin A2 to C2, and the enzyme is found in human serum Polet_H et al. and in the plasma of rabbit, cat, pig, dog and rat Jones_RL et al.. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER; m/e 348(M+), 330, 249, 245, 217, 215, 190, 133, 119, 109 Floyd_MB et al. |
| UV Spectra | λ MeOH max = 234 nm (ε ∼ 17000) Corey_EJ et al. |
| IR Spectra | CHLOROFORM solution, ν 1750,1715 cm-1 Corey_EJ et al. |
| NMR Spectra | METHYL ESTER ; δ 6.3(d, J=16Hz, 1H, 13-CH), 6.1-5.95(m, 2H, 11-CH), 5,7(dd, J=6,16Hz, 1H, 14-CH), 5.7-5.1(m, 2H, 5,6-CH), 4.4-4.0(m, 1H, 15-CH), 3.69(S, 3H, OCH3), 3.3-3.0(m, 1H,8-CH), 3.0-2.8(m, 2H, 10-CH) Floyd_MB et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|