LBF20307PG38: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&Prostaglandin J_2&& | |Common Name=&&Prostaglandin J_2&& | ||
|Solubility=ACETONITRILE<!--以下1048-->[[Reference:Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093| | |Solubility=ACETONITRILE<!--以下1048-->[[Reference:Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093| | ||
{{RelationTable/GetFirstAuthor|Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093}}]] | {{RelationTable/GetFirstAuthor|Reference:Mubarik-Ali_S:Chapeleo_CB:Finch_MAW:Roberts_SM:Woolley_GT:Cave_RJ:and Newton_RF:,J. Chem. Soc.,1980,,2093}}]], CHLOROFORM, ETHANOL [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
, CHLOROFORM, ETHANOL [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | |||
|Mass Spectra=TMS ETHER ; M^+ 478.2934 [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | |Mass Spectra=TMS ETHER ; M^+ 478.2934 [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
|UV Spectra= lambda ^{MeOH}_{max} = 305( epsilon 1200), 216( epsilon 9900)nm [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | |UV Spectra= lambda ^{MeOH}_{max} = 305( epsilon 1200), 216( epsilon 9900)nm [[Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Morton_DR:Peterson_DC:Nishizawa_EE:Miller_WL:,J. Med. Chem.,1983,26,790}}]] | ||
Revision as of 06:04, 12 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1901 |
| LipidMaps | LMFA03010019 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG38 |
| Prostaglandin J2 | |
|---|---|
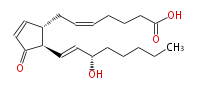
| |
| Structural Information | |
| 7- [ 2 (R) - (3 (S) -Hydroxy-1 (E) -octenyl) -3-oxo-4-cyclopenten-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| PGJ2 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)C=C[C@@H](C(=O)1)[C@@H](CC=CCCCC(O)=O)C=C1)CC |
| Physicochemical Information | |
| ACETONITRILE Mubarik-AliSet al., CHLOROFORM, ETHANOL Bundy_GL et al. | |
Bundy_GL et al.  | |
| In aqueous solution prostaglandin D2 undergoes non-enzymatic dehydration and is converted to prostaglandin J2 Fukushima_M . | |
| The anti-tumor and anti-viral activities of prostaglandin J2 are attributed to Delta 12-prostaglandin J2 which is a degradation product of prostagladnin J2 and is characteristic of its alkylidene cyclopentenone structure Fukushima_M . | |
| Spectral Information | |
| Mass Spectra | TMS ETHER ; M+ 478.2934 Bundy_GL et al. |
| UV Spectra | λ MeOH max = 305( ε 1200), 216( ε 9900)nm Bundy_GL et al. |
| IR Spectra | ν 3400, 3200, 2660, 1710, 1085, 970 cm-1 Bundy_GL et al. |
| NMR Spectra | 1H-NMR(CDCl3) : δ 7.75-7.55(m, 1H, 9-CH), 6.30-6.10(m, 1H, 10-CH), 5.90(brs, 2H, OH), 5.75-5.35(m, 4H), 4.30-3.95(m, 1H, 15-CH) Bundy_GL et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|