LBF20307PG41: Difference between revisions
No edit summary |
No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR8054 | |LipidBank=XPR8054 | ||
|LipidMaps=LMFA03010100 | |LipidMaps=LMFA03010100 | ||
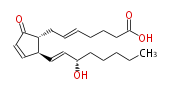
|SysName= ( | |SysName=(trans-5,trans-13)-(8R,12S,15S)-15-Hydroxy-9-oxoprost-5,10,13-trienoic acid | ||
|Common Name=&&5-trans- | |Common Name=&&5-trans- Prostaglandin A_2&&(5E,13E)-(8R,12S,15S)-15-Hydroxy-9-oxoprost-5,10,13-trienoic acid&& | ||
| | |Optical=[ alpha ]_D +128°(CHCl_3 )[[Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124}}]] | ||
|Mass Spectra=HREIMS m/z 478.2998 for TMS derivative | |Mass Spectra=HREIMS m/z 478.2998 for TMS derivative C_{26}H_{46}O_4 Si_2 , calcd 478.2932.[[Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124}}]] | ||
|UV Spectra= | |UV Spectra= lambda _{max} 217 nm( epsilon 9050))[[Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124}}]] | ||
|Source=5-trans-Prostaglandin | |Source=5-trans-Prostaglandin A_2 was isolated from Gorgonian, Plexaura homomalla.[[Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Daniels_EG:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2124}}]] | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Symbol=5-trans- PGA_2 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 09:03, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8054 |
| LipidMaps | LMFA03010100 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20307PG41 |
| 5-trans- Prostaglandin A2 | |
|---|---|
| |
| Structural Information | |
| (trans-5,trans-13)-(8R,12S,15S)-15-Hydroxy-9-oxoprost-5,10,13-trienoic acid | |
| |
| 5-trans- PGA_2 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)C=CC(=O)1)CC |
| Physicochemical Information | |
| [ α ]D +128°(CHCl3) Bundy_GL et al. | |
| 5-trans-Prostaglandin A_2 was isolated from Gorgonian, Plexaura homomalla. Bundy_GL et al. | |
| Spectral Information | |
| Mass Spectra | HREIMS m/z 478.2998 for TMS derivative C26}H_{46O4 Si2, calcd 478.2932. Bundy_GL et al. |
| UV Spectra | λ max 217 nm( ε 9050)) Bundy_GL et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|