LBF20308PG03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName=7- [ 2 (E) - (3 (S) -Hydroxyoctylidene) -3-oxo-4-cyclopenten-1 (R) -yl ] -5 (Z) -heptenoic acid | |SysName=7- [ 2 (E) - (3 (S) -Hydroxyoctylidene) -3-oxo-4-cyclopenten-1 (R) -yl ] -5 (Z) -heptenoic acid | ||
|Solubility=METHANOL | |Solubility=METHANOL[[Reference:Fitzpatrick_FA:Wynalda_MA:,J. Biol. Chem.,1983,258,11713|{{RelationTable/GetFirstAuthor|Reference:Fitzpatrick_FA:Wynalda_MA:,J. Biol. Chem.,1983,258,11713}}]]ETHANOL, CHLOROFORM, ETHYL ACETATE [[Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317|{{RelationTable/GetFirstAuthor|Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317}}]] | ||
|Mass Spectra=m/e 334(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 316, 245, 236 | |Mass Spectra=m/e 334(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 316, 245, 236 [[Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317|{{RelationTable/GetFirstAuthor|Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>E</FONT></SUP><SUP><FONT SIZE=-1>t</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 244(<FONT FACE="Symbol">e</FONT> 6100)nm | |UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>E</FONT></SUP><SUP><FONT SIZE=-1>t</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 244(<FONT FACE="Symbol">e</FONT> 6100)nm [[Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317|{{RelationTable/GetFirstAuthor|Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317}}]] | ||
|IR Spectra=<FONT FACE="Symbol">n</FONT> : 2930, 1700, 1640, 1580, 1232, 028 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=<FONT FACE="Symbol">n</FONT> : 2930, 1700, 1640, 1580, 1232, 028 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317|{{RelationTable/GetFirstAuthor|Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 7.5(dd, 1H, 9-CH), 6.56(t, 1H, 13-CH), 6.35(dd,1 H, 10-CH), 5.48(m, 2H, 5,6-CH), 3.88(m, 1H, 15-CH), 3.44(m, 1H, 8-CH) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 7.5(dd, 1H, 9-CH), 6.56(t, 1H, 13-CH), 6.35(dd,1 H, 10-CH), 5.48(m, 2H, 5,6-CH), 3.88(m, 1H, 15-CH), 3.44(m, 1H, 8-CH) [[Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317|{{RelationTable/GetFirstAuthor|Reference:Kikawa_Y:Narumiya_S:Fukushima_M:Wakatsuka_H:Hayaishi_O:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,1317}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1911 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20308PG03 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
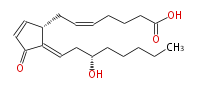
| 7- [ 2 (E) - (3 (S) -Hydroxyoctylidene) -3-oxo-4-cyclopenten-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC[C@@H](O)CC=C(C(=O)1)[C@@H](CC=CCCCC(O)=O)C=C1)CC |
| Physicochemical Information | |
| METHANOL Fitzpatrick_FA et al.ETHANOL, CHLOROFORM, ETHYL ACETATE KikawaYet al. | |
| Spectral Information | |
| Mass Spectra | m/e 334(M+), 316, 245, 236 KikawaYet al. |
| UV Spectra | l EtOHmax = 244(e 6100)nm KikawaYet al. |
| IR Spectra | n : 2930, 1700, 1640, 1580, 1232, 028 cm-1 KikawaYet al. |
| NMR Spectra | 1H-NMR(CDCl3) : d 7.5(dd, 1H, 9-CH), 6.56(t, 1H, 13-CH), 6.35(dd,1 H, 10-CH), 5.48(m, 2H, 5,6-CH), 3.88(m, 1H, 15-CH), 3.44(m, 1H, 8-CH) KikawaYet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|