LBF20309HO02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=DFA8142 | |LipidBank=DFA8142 | ||
|LipidMaps=LMFA03050005 | |LipidMaps=LMFA03050005 | ||
|SysName=5S- | |SysName=5S-Hydroxy- (6-trans,8-cis,11-cis) -icosatrienoic acid | ||
|Common Name=&&5S- | |Common Name=&&5S-Hydroxy- (6E,8Z,11Z) -eicosatrienoic acid&& | ||
|UV Spectra= lambda max: 235nm epsilon : 23,000 | |UV Spectra= lambda max: 235nm epsilon : 23,000 | ||
|Source=5(S)-HETrE is produced by the action of 5-lipoxygenase when mead acid is the substrate [[Reference:Jakschik_BA:Sams_AR:Sprecher_H:Needleman_P:,Prostaglandins,1980,20,401|{{RelationTable/GetFirstAuthor|Reference:Jakschik_BA:Sams_AR:Sprecher_H:Needleman_P:,Prostaglandins,1980,20,401}}]][[Reference:Jakschik_BA:Morrison_AR:Sprecher_H:,J. Biol. Chem.,1983,258,12797|{{RelationTable/GetFirstAuthor|Reference:Jakschik_BA:Morrison_AR:Sprecher_H:,J. Biol. Chem.,1983,258,12797}}]]. | |Source=5(S)-HETrE is produced by the action of 5-lipoxygenase when mead acid is the substrate [[Reference:Jakschik_BA:Sams_AR:Sprecher_H:Needleman_P:,Prostaglandins,1980,20,401|{{RelationTable/GetFirstAuthor|Reference:Jakschik_BA:Sams_AR:Sprecher_H:Needleman_P:,Prostaglandins,1980,20,401}}]][[Reference:Jakschik_BA:Morrison_AR:Sprecher_H:,J. Biol. Chem.,1983,258,12797|{{RelationTable/GetFirstAuthor|Reference:Jakschik_BA:Morrison_AR:Sprecher_H:,J. Biol. Chem.,1983,258,12797}}]]. | ||
Revision as of 06:46, 28 May 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8142 |
| LipidMaps | LMFA03050005 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20309HO02 |
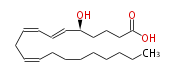
| 5S-Hydroxy- (6E,8Z,11Z) -eicosatrienoic acid | |
|---|---|
| |
| Structural Information | |
| 5S-Hydroxy- (6-trans,8-cis,11-cis) -icosatrienoic acid | |
| |
| 5(S)-HETrE | |
| Formula | C20H34O3 |
| Exact Mass | 322.25079495399996 |
| Average Mass | 322.48216 |
| SMILES | C(CCCCC=CCC=CC=CC(CCCC(O)=O)O)CCC |
| Physicochemical Information | |
| 5(S)-HETrE is produced by the action of 5-lipoxygenase when mead acid is the substrate Jakschik_BA et al. Jakschik_BA et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 235nm ε : 23,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|