LBF20406AM06: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA08020008 | |LipidMaps=LMFA08020008 | ||
|SysName=arachidonoyl amide | |SysName=arachidonoyl amide | ||
|Common Name=&&arachidonoyl amide&& | |||
|Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Melting Point=colorless oil [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.82 (br s, lH), 5.31-5.42 (m, 8H), 2.79-2.85 (m, 6H), 2.23 (t, J=8.1Hz, 2H), 2.04-2.15 (m, 4H), 1.70-1.77 (m, 2H), 1.25-1.38 (m, 6H), 0.89 (t, J=6.8Hz,3H). [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.82 (br s, lH), 5.31-5.42 (m, 8H), 2.79-2.85 (m, 6H), 2.23 (t, J=8.1Hz, 2H), 2.04-2.15 (m, 4H), 1.70-1.77 (m, 2H), 1.25-1.38 (m, 6H), 0.89 (t, J=6.8Hz,3H). [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
}} | }} | ||
Revision as of 00:01, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR7022 |
| LipidMaps | LMFA08020008 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406AM06 |
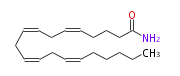
| arachidonoyl amide | |
|---|---|
| |
| Structural Information | |
| arachidonoyl amide | |
| |
| Formula | C20H33NO |
| Exact Mass | 303.256214683 |
| Average Mass | 303.48215999999996 |
| SMILES | C(CC=CCC=CCC=CCC=CCCCC(N)=O)CCC |
| Physicochemical Information | |
| colorless oil Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) d5.82 (br s, lH), 5.31-5.42 (m, 8H), 2.79-2.85 (m, 6H), 2.23 (t, J=8.1Hz, 2H), 2.04-2.15 (m, 4H), 1.70-1.77 (m, 2H), 1.25-1.38 (m, 6H), 0.89 (t, J=6.8Hz,3H). SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|